The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer's disease: a longitudinal PET study
- PMID: 32375809
- PMCID: PMC7203856
- DOI: 10.1186/s12974-020-01820-6
The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer's disease: a longitudinal PET study
Abstract
Background: The aim of this longitudinal study was to assess with positron emission tomography (PET) the relationship between levels of inflammation and the loads of aggregated β-amyloid and tau at baseline and again after 2 years in prodromal Alzheimer's disease.
Methods: Forty-three subjects with mild cognitive impairment (MCI) had serial 11C-PK11195 PET over 2 years to measure inflammation changes, and 11C-PiB PET to determine β-amyloid fibril load; 22 also had serial 18F-Flortaucipir PET to determine tau tangle load. Cortical surface statistical mapping was used to localise areas showing significant changes in tracer binding over time and to interrogate correlations between tracer binding of the tracers at baseline and after 2 years.
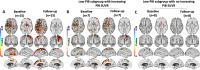
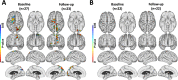
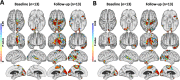
Results: Those MCI subjects with high 11C-PiB uptake at baseline (classified as prodromal Alzheimer's disease) had raised inflammation levels which significantly declined across cortical regions over 2 years although their β-amyloid levels continued to rise. Those MCI cases who had low/normal 11C-PiB uptake at baseline but their levels then rose over 2 years were classified as prodromal AD with low Thal phase 1-2 amyloid deposition at baseline. They showed levels of cortical inflammation which correlated with their rising β-amyloid load. Those MCI cases with baseline low 11C-PiB uptake that remained stable were classified as non-AD, and they showed no correlated inflammation levels. Finally, MCI cases which showed both high 11C-PiB and 18F-Flortaucipir uptake at baseline (MCI due to AD) showed a further rise in their tau tangle load over 2 years with a correlated rise in levels of inflammation.
Conclusions: Our baseline and 2-year imaging findings are compatible with a biphasic trajectory of inflammation in Alzheimer's disease: MCI cases with low baseline but subsequently rising β-amyloid load show correlated levels of microglial activation which then later decline when the β-amyloid load approaches AD levels. Later, as tau tangles form in β-amyloid positive MCI cases with prodromal AD, the rising tau load is associated with higher levels of inflammation.
Keywords: Alzheimer; Flortaucipir; MCI; Microglia; Neuroinflammation; PET; PK11195; PiB; Tau; β-amyloid.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Serrano-Pozo A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol [Internet]. 2011 Sep [cited 2020 Jan 21];179(3):1373–84. Available from: 10.1016/j.ajpath.2011.05.047. - PMC - PubMed
-
- Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimer’s Dement [Internet]. 2016 Jun 1 [cited 2018 Dec 11];12(6):719–32. Available from: 10.1016/j.jalz.2016.02.010. - PubMed
-
- Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences [Internet]. Vol. 79, Neuroscience Research. 2014 [cited 2019 Mar 13]. p. 1–12. Available from: 10.1016/j.neures.2013.10.004. - PubMed
-
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol [Internet]. 2013 [cited 2019 Feb 25];12(4):357–67. Available from: http://dx.doi.org/10.1016/. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

