Spot the Difference: Function versus Toxicity in Amyloid Fibrils
- PMID: 32376150
- PMCID: PMC7617689
- DOI: 10.1016/j.tibs.2020.04.007
Spot the Difference: Function versus Toxicity in Amyloid Fibrils
Abstract
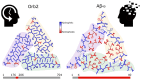
In a recent study, Hervas et al. extracted Orb2 fibrils, that are involved in long-term memory formation, from Drosophila brains, characterised their function, and determined their structure using cryo-electron microscopy (cryo-EM). The fibrils show a remarkable resemblance to amyloid β (Aβ) fibrils associated with Alzheimer's disease, highlighting the subtle difference between functional and dysfunctional amyloid.
Keywords: Alzheimer; CPEB; Orb2; amyloid; cryo-electron microscopy; memory.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment on
-
Cryo-EM structure of a neuronal functional amyloid implicated in memory persistence in Drosophila.Science. 2020 Mar 13;367(6483):1230-1234. doi: 10.1126/science.aba3526. Science. 2020. PMID: 32165583 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

