[Effect of heat inactivation of blood samples on the efficacy of three detection methods of SARS-CoV-2 antibodies]
- PMID: 32376571
- PMCID: PMC7167315
- DOI: 10.12122/j.issn.1673-4254.2020.03.03
[Effect of heat inactivation of blood samples on the efficacy of three detection methods of SARS-CoV-2 antibodies]
Abstract
Objective: To evaluate the effects of heat inactivation of blood samples at 56℃ for 30 min on the results of SARS-CoV-2 antibody detection using different methods.
Methods: This retrospective study was conducted in 11 patients with established diagnosis of COVID-19 and 10 patients with diseases other than COVID- 19 in our hospital. We collected samples of serum, plasma and whole blood from each patient between February, 12 and 18, 2020, and with a double- blind design, the samples were examined for SARS-CoV-2 antibodies before and after heat inactivation at 56 ℃ for 30 min. In all the samples, the total SARS-CoV-2 antibodies were detected using immunochromatography, and the IgM antibodies were detected using fluorescence immunochromatography; the IgM and IgG antibodies in the serum and plasma samples detected with chemiluminescence immunoassay. We compared the detection results and analyzed the correlation of semi-quantitative detection results of IgM and IgG antibodies before and after heat inactivation of the samples.
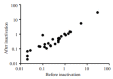
Results: With immuno-chromatography, the coincidence rate of the total SARS-CoV-2 antibody detection before and after heat inactivation of the serum and plasma samples was 90.0% in COVID-19 cases and 100.0% in the negative cases, resulting in a total coincidence rate 95.2%; for the whole blood samples, the total coincidence rates of the total SARS-CoV-2 antibodies were 100.0%. For detection of IgM antibodies in the serum, plasma and whole blood samples using fluorescence immunochromatography, the coincidence rates in SARS-CoV-2-positive and negative cases and the total coincidence rate before and after inactivation were 100.0%, 0 and 47.6%, respectively. For detection of serum IgM and IgG antibodies and plasma IgG antibodies with chemiluminescence immunoassay, the coincidence rates in SARS-CoV-2-positive and negative cases and the total coincidence rate before and after inactivation were all 100.0%, and the total coincidence rate of plasma IgM antibodies was 95.2%. Pearson correlation analysis of the semi-quantitative results of IgM and IgG detection in the serum and plasma samples showed a correlation coefficient of 0.9999 (95%CI: 0.9998-1.000, P < 0.001) between the results before and after sample inactivation.
Conclusions: Heat inactivation of blood samples at 56 ℃ for 30 min does not obviously affect the results of immunochromatography and chemiluminescent immunoassay for detection of SARS-COV-2 antibodies but can reduce the risk of infection for the operators. Heat-inactivated samples can not be used in fluorescence immunochromatography for SARS-CoV-2 antibody detection.
目的: 探讨56 ℃ 30 min灭活处理对不同方法测定2019新型冠状病毒(SARS-CoV-2)抗体检测结果的影响。
方法: 本文为回顾性研究。收集2020年2月12~18日在佛山市第一人民医院确诊为新型冠状病毒性肺炎患者血清样本、血浆样本及全血样本各11份, 同时收集同期疑似病例及其他疾病患者血清、血浆及全血样本各10份, 采用双盲法, 在样本采用56℃ 30 min灭活处理前后, 分别采用免疫层析法检测样本中的SARS-CoV-2总抗体、采用荧光免疫层析法检测样本中的SARS-CoV-2 IgM抗体、采用化学发光荧光免疫分析法检测血清及血浆样本SARS-CoV-2 IgM及SARS-CoV-2 IgG, 比较灭活前与灭活处理后样本的抗体检测结果, 并采用Pearson相关性分析灭活前后检测IgM及IgG半定量检测结果的相关性。
结果: 免疫层析法检测灭活前后血清与血浆样本中SARS-CoV-2总抗体的阳性样本符合率为90.9%, 阴性符合率为100.0%, 总符合率均为95.2%。免疫层析法检测灭活前后全血样本中SARS-CoV-2总抗体的总符合率为100.0%。荧光免疫层析法检测灭活前后血清、血浆及全血样本中SARS-CoV-2 IgM抗体的阳性符合率均为100.0%, 阴性符合率均为0%, 总符合率均为47.6%。化学发光免疫分析法检测灭活前后血清样本中SARS-CoV-2 IgM及IgG抗体及血浆中的IgG抗体, 阳性符合率、阴性符合率及总符合率均为100.0%, 检测血浆IgM抗体的总符合率为95.2%。采用Pearson相关对灭活前后血清及血浆样本IgM和IgG半定量检测结果进行相关性分析, 其中IgM灭活前后结果的相关系数为0.9999(95%CI:0.9998~1.000, P < 0.001), IgG灭活前后结果的相关系数为0.9999(95%CI:0.9998~1.000, P < 0.001)。
结论: 56 ℃ 30 min灭活处理血液样本对免疫层析法及化学发光免疫分析法检测SARS-CoV-2抗体结果几乎无影响, 可在检测前先进行灭活以降低检验人员感染风险, 荧光免疫层析法不可使用灭活后样本进行检测。
Keywords: SARS-CoV-2; antibodies; heat inactivation; plasma; serum.
Figures
References
-
- Nishiura H, Jung SM, Linton NM, et al. The extent of transmission of novel coronavirus in wuhan, China, 2020. J Clin Med. 2020;24(9):pii: E330. doi: 10.3390/jcm9020330. [Nishiura H, Jung SM, Linton NM, et al. The extent of transmission of novel coronavirus in wuhan, China, 2020[J]. J Clin Med, 2020, 24 (9). pii: E330. DOI: 10.3390/jcm9020330.] - DOI - PMC - PubMed
-
- HuiDS, I AzharE, MadaniTA, et al. The continuing 2019-nCoV epi-demic threat of novel coronaviruses to global health-the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–6. doi: 10.1016/j.ijid.2020.01.009. [HuiDS, I AzharE, MadaniTA, et al. The continuing 2019-nCoV epi-demic threat of novel coronaviruses to global health-the latest 2019 novel coronavirus outbreak in Wuhan, China[J]. Int J Infect Dis, 2020, 91: 264-6. DOI: 10.1016/j.ijid.2020.01.009.] - DOI - PMC - PubMed
-
-
关于印发医疗机构内新型冠状病毒感染预防与控制技术指南(第一版)的通知(.国卫办医函[2020]65号)[EB/OL].2020. <a href="http://www.nhc.gov.cn/yzygj/s7659/202001/b91fdab7c304431eb082d67847d27e14.shtml" target="_blank">http://www.nhc.gov.cn/yzygj/s7659/202001/b91fdab7c304431eb082d67847d27e14.shtml</a>.
-
-
- 华 文浩, 盛 琳君, 宋 丽红, et al. 生物安全Ⅱ级实验室开展新型冠状病毒感染患者实验检测的风险评估与防控. 中华检验医学杂志. 2020;43(00):E007–E007. doi: 10.3760/cma.j.issn.1009-9158.2010.0007. doi: 10.3760/cma.j.issn.1009-9158.2010.0007. [华文浩, 盛琳君, 宋丽红, 等.生物安全Ⅱ级实验室开展新型冠状病毒感染患者实验检测的风险评估与防控[J].中华检验医学杂志, 2020, 43(00): E007-E007. DOI: 10.3760/cma.j.issn.1009-9158.2010.0007.] - DOI
-
- 陈 培松, 何 宇婷, 黄 裕立, et al. 不同方式灭活口咽拭子标本对2019新型冠状病毒实时荧光定量PCR检测结果的影响. 中华检验医学杂志. 2020;43(00):E004–E004. doi: 10.3760/cma.j.issn.1009-9158.2020.0004. doi: 10.3760/cma.j.issn.1009-9158.2020.0004. [陈培松, 何宇婷, 黄裕立, 等.不同方式灭活口咽拭子标本对2019新型冠状病毒实时荧光定量PCR检测结果的影响[J].中华检验医学杂志, 2020, 43(00): E004-E004. DOI: 10.3760/cma.j.issn.1009-9158.2020.0004.] - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous