Pattern of Invasion in Human Pancreatic Cancer Organoids Is Associated with Loss of SMAD4 and Clinical Outcome
- PMID: 32376602
- PMCID: PMC7335355
- DOI: 10.1158/0008-5472.CAN-19-1523
Pattern of Invasion in Human Pancreatic Cancer Organoids Is Associated with Loss of SMAD4 and Clinical Outcome
Abstract
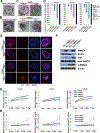
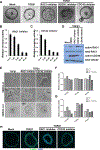
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy characterized by extensive local invasion and systemic spread. In this study, we employed a three-dimensional organoid model of human pancreatic cancer to characterize the molecular alterations critical for invasion. Time-lapse microscopy was used to observe invasion in organoids from 25 surgically resected human PDAC samples in collagen I. Subsequent lentiviral modification and small-molecule inhibitors were used to investigate the molecular programs underlying invasion in PDAC organoids. When cultured in collagen I, PDAC organoids exhibited two distinct, morphologically defined invasive phenotypes, mesenchymal and collective. Each individual PDAC gave rise to organoids with a predominant phenotype, and PDAC that generated organoids with predominantly mesenchymal invasion showed a worse prognosis. Collective invasion predominated in organoids from cancers with somatic mutations in the driver gene SMAD4 (or its signaling partner TGFBR2). Reexpression of SMAD4 abrogated the collective invasion phenotype in SMAD4-mutant PDAC organoids, indicating that SMAD4 loss is required for collective invasion in PDAC organoids. Surprisingly, invasion in passaged SMAD4-mutant PDAC organoids required exogenous TGFβ, suggesting that invasion in SMAD4-mutant organoids is mediated through noncanonical TGFβ signaling. The Rho-like GTPases RAC1 and CDC42 acted as potential mediators of TGFβ-stimulated invasion in SMAD4-mutant PDAC organoids, as inhibition of these GTPases suppressed collective invasion in our model. These data suggest that PDAC utilizes different invasion programs depending on SMAD4 status, with collective invasion uniquely present in PDAC with SMAD4 loss. SIGNIFICANCE: Organoid models of PDAC highlight the importance of SMAD4 loss in invasion, demonstrating that invasion programs in SMAD4-mutant and SMAD4 wild-type tumors are different in both morphology and molecular mechanism.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

