Development of an inactivated vaccine candidate for SARS-CoV-2
- PMID: 32376603
- PMCID: PMC7202686
- DOI: 10.1126/science.abc1932
Development of an inactivated vaccine candidate for SARS-CoV-2
Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in an unprecedented public health crisis. Because of the novelty of the virus, there are currently no SARS-CoV-2-specific treatments or vaccines available. Therefore, rapid development of effective vaccines against SARS-CoV-2 are urgently needed. Here, we developed a pilot-scale production of PiCoVacc, a purified inactivated SARS-CoV-2 virus vaccine candidate, which induced SARS-CoV-2-specific neutralizing antibodies in mice, rats, and nonhuman primates. These antibodies neutralized 10 representative SARS-CoV-2 strains, suggesting a possible broader neutralizing ability against other strains. Three immunizations using two different doses, 3 or 6 micrograms per dose, provided partial or complete protection in macaques against SARS-CoV-2 challenge, respectively, without observable antibody-dependent enhancement of infection. These data support the clinical development and testing of PiCoVacc for use in humans.
Copyright © 2020, American Association for the Advancement of Science.
Figures


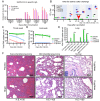
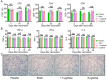
Comment in
-
Impaired interferon signature in severe COVID-19.Nat Rev Immunol. 2020 Jun;20(6):353. doi: 10.1038/s41577-020-0335-0. Nat Rev Immunol. 2020. PMID: 32355328 Free PMC article. No abstract available.
-
SARS-CoV-2-reactive T cells in patients and healthy donors.Nat Rev Immunol. 2020 Jun;20(6):353. doi: 10.1038/s41577-020-0333-2. Nat Rev Immunol. 2020. PMID: 32355329 Free PMC article. No abstract available.
-
Inactivated vaccine for SARS-CoV-2.Nat Rev Immunol. 2020 Jun;20(6):353. doi: 10.1038/s41577-020-0334-1. Nat Rev Immunol. 2020. PMID: 32355330 Free PMC article. No abstract available.
-
Coronavirus vaccine trials have delivered their first results - but their promise is still unclear.Nature. 2020 May;581(7809):363-364. doi: 10.1038/d41586-020-01092-3. Nature. 2020. PMID: 32433634 No abstract available.
References
-
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T., Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 27, 325–328 (2020). 10.1016/j.chom.2020.02.001 - DOI - PMC - PubMed
-
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y. S., Singh K. P., Chaicumpa W., COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin. Immunother. 10.1080/21645515.2020.1735227 (2020). 10.1080/21645515.2020.1735227 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

