Proteomics of Campylobacter jejuni Growth in Deoxycholate Reveals Cj0025c as a Cystine Transport Protein Required for Wild-type Human Infection Phenotypes
- PMID: 32376616
- PMCID: PMC8015009
- DOI: 10.1074/mcp.RA120.002029
Proteomics of Campylobacter jejuni Growth in Deoxycholate Reveals Cj0025c as a Cystine Transport Protein Required for Wild-type Human Infection Phenotypes
Abstract
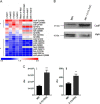
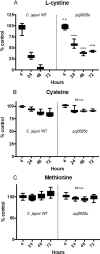
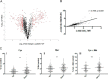
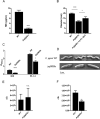
Campylobacter jejuni is a major cause of food-borne gastroenteritis. Proteomics by label-based two-dimensional liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) identified proteins associated with growth in 0.1% sodium deoxycholate (DOC, a component of gut bile salts), and system-wide validation was performed by data-independent acquisition (DIA-SWATH-MS). LC-MS/MS quantified 1326 proteins (∼82% of the predicted C. jejuni proteome), of which 1104 were validated in additional biological replicates by DIA-SWATH-MS. DOC resulted in a profound proteome shift with 512 proteins showing significantly altered abundance. Induced proteins were associated with flagellar motility and antibiotic resistance; and these correlated with increased DOC motility and resistance to polymyxin B and ciprofloxacin. DOC also increased human Caco-2 cell adherence and invasion. Abundances of proteins involved in nutrient transport were altered by DOC and aligned with intracellular changes to their respective carbon sources. DOC increased intracellular levels of sulfur-containing amino acids (cysteine and methionine) and the dipeptide cystine (Cys-Cys), which also correlated with reduced resistance to oxidative stress. A DOC induced transport protein was Cj0025c, which has sequence similarity to bacterial Cys-Cys transporters. Deletion of cj0025c (Δcj0025c) resulted in proteome changes consistent with sulfur starvation, as well as attenuated invasion, reduced motility, atypical morphology, increased antimicrobial susceptibility and poor biofilm formation. Targeted metabolomics showed Δcj0025c could use known C. jejuni amino and organic acid substrates commensurate with wild-type. Medium Cys-Cys levels however, were maintained in Δcj0025c relative to wild-type. A toxic Cys-Cys mimic (selenocystine) inhibited wild-type growth, but not Δcj0025c Provision of an alternate sulfur source (2 mm thiosulfate) restored Δcj0025c motility. Our data confirm that Cj0025c is a Cys-Cys transporter that we have named TcyP consistent with the nomenclature of homologous proteins in other species.
Keywords: Bacteria; Bacterial pathogenesis; Campylobacter jejuni; Cystine; Mass Spectrometry; Metabolomics; Nutrient transport; Pathogens; Sulfur; Virulence.
© 2020 Man et al.
Conflict of interest statement
Conflict of interest—Authors declare no competing interests.
Figures





References
-
- Hermans D., Pasmans F., Heyndrickx M., Van Immerseel F., Martel A., Van Deun K., Haesebrouck F. A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut. Crit. Rev. Microbiol. 2012;38:17–29. - PubMed
-
- Awad W.A., Hess C., Hess M. Re-thinking chicken-Campylobacter jejuni interaction: a review. Avian Pathol. 2018;47:352–363. - PubMed
-
- Black R.E., Levine M.M., Clements M.L., Hughes T.P., Blaser M.J. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988;157:472–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

