Sonic hedgehog specifies flight feather positional information in avian wings
- PMID: 32376617
- PMCID: PMC7225127
- DOI: 10.1242/dev.188821
Sonic hedgehog specifies flight feather positional information in avian wings
Abstract
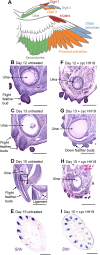
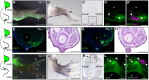
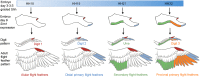
Classical tissue recombination experiments performed in the chick embryo provide evidence that signals operating during early limb development specify the position and identity of feathers. Here, we show that Sonic hedgehog (Shh) signalling in the embryonic chick wing bud specifies positional information required for the formation of adult flight feathers in a defined spatial and temporal sequence that reflects their different identities. We also reveal that Shh signalling is interpreted into specific patterns of Sim1 and Zic transcription factor expression, providing evidence of a putative gene regulatory network operating in flight feather patterning. Our data suggest that flight feather specification involved the co-option of the pre-existing digit patterning mechanism and therefore uncovers an embryonic process that played a fundamental step in the evolution of avian flight.
Keywords: Avian; Chick; Embryo; Flight feather; Positional information; Shh.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures






References
-
- Cairns J. M. and Saunders J. R. (1954). The influence of embryonic mesoderm on the regional specification of epidermal derivatives in the chick. J. Exp. Zool. 127, 221-248. 10.1002/jez.1401270203 - DOI

