A C3-specific nanobody that blocks all three activation pathways in the human and murine complement system
- PMID: 32376685
- PMCID: PMC7324514
- DOI: 10.1074/jbc.RA119.012339
A C3-specific nanobody that blocks all three activation pathways in the human and murine complement system
Abstract
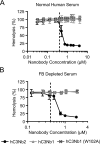
The complement system is a tightly controlled proteolytic cascade in the innate immune system, which tags intruding pathogens and dying host cells for clearance. An essential protein in this process is complement component C3. Uncontrolled complement activation has been implicated in several human diseases and disorders and has spurred the development of therapeutic approaches that modulate the complement system. Here, using purified proteins and several biochemical assays and surface plasmon resonance, we report that our nanobody, hC3Nb2, inhibits C3 deposition by all complement pathways. We observe that the hC3Nb2 nanobody binds human native C3 and its degradation products with low nanomolar affinity and does not interfere with the endogenous regulation of C3b deposition mediated by Factors H and I. Using negative stain EM analysis and functional assays, we demonstrate that hC3Nb2 inhibits the substrate-convertase interaction by binding to the MG3 and MG4 domains of C3 and C3b. Furthermore, we notice that hC3Nb2 is cross-reactive and inhibits the lectin and alternative pathway in murine serum. We conclude that hC3Nb2 is a potent, general, and versatile inhibitor of the human and murine complement cascades. Its cross-reactivity suggests that this nanobody may be valuable for analysis of complement activation within animal models of both acute and chronic diseases.
Keywords: C3 convertase; antibody; complement system; inhibitor; innate immunity; nanobody; single-domain antibody; single-domain antibody (sdAb nanobody); structural biology.
© 2020 Pedersen et al.
Conflict of interest statement
Conflict of interest—G. R. A. has a collaboration with Alexion pharmaceuticals.
Figures




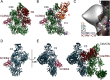
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

