Copper stabilizes antiparallel β-sheet fibrils of the amyloid β40 (Aβ40)-Iowa variant
- PMID: 32376688
- PMCID: PMC7335782
- DOI: 10.1074/jbc.RA119.011955
Copper stabilizes antiparallel β-sheet fibrils of the amyloid β40 (Aβ40)-Iowa variant
Abstract
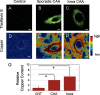
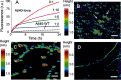
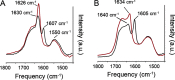
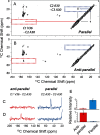
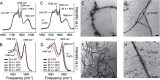
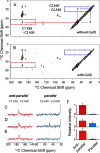
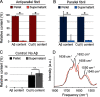
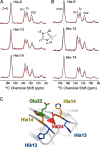
Cerebral amyloid angiopathy (CAA) is a vascular disorder that primarily involves deposition of the 40-residue-long β-amyloid peptide (Aβ40) in and along small blood vessels of the brain. CAA is often associated with Alzheimer's disease (AD), which is characterized by amyloid plaques in the brain parenchyma enriched in the Aβ42 peptide. Several recent studies have suggested a structural origin that underlies the differences between the vascular amyloid deposits in CAA and the parenchymal plaques in AD. We previously have found that amyloid fibrils in vascular amyloid contain antiparallel β-sheet, whereas previous studies by other researchers have reported parallel β-sheet in fibrils from parenchymal amyloid. Using X-ray fluorescence microscopy, here we found that copper strongly co-localizes with vascular amyloid in human sporadic CAA and familial Iowa-type CAA brains compared with control brain blood vessels lacking amyloid deposits. We show that binding of Cu(II) ions to antiparallel fibrils can block the conversion of these fibrils to the more stable parallel, in-register conformation and enhances their ability to serve as templates for seeded growth. These results provide an explanation for how thermodynamically less stable antiparallel fibrils may form amyloid in or on cerebral vessels by using Cu(II) as a structural cofactor.
Keywords: Abeta40-Iowa; Alzheimer disease; Alzheimer's disease; Aβ40-Iowa; Fourier transform IR (FTIR); NMR spectroscopy; X-ray fluorescence microscopy (XFM); amyloid-beta (AB); cerebral amyloid angiopathy (CAA,); copper; fibril; metal ions; neurodegeneration; vascular disease.
Conflict of interest statement
Conflict of interest—The authors declare no competing financial interests.
Figures

References
-
- Qi-Takahara Y., Morishima-Kawashima M., Tanimura Y., Dolios G., Hirotani N., Horikoshi Y., Kametani F., Maeda M., Saido T. C., Wang R., and Ihara Y. (2005) Longer forms of amyloid β protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J. Neurosci. 25, 436–445 10.1523/JNEUROSCI.1575-04.2005 - DOI - PMC - PubMed
-
- Castaño E. M., Prelli F., Soto C., Beavis R., Matsubara E., Shoji M., and Frangione B. (1996) The length of amyloid-β in hereditary cerebral hemorrhage with amyloidosis, Dutch type: implications for the role of amyloid-β 1-42 in Alzheimer's disease. J. Biol. Chem. 271, 32185–32191 10.1074/jbc.271.50.32185 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

