BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis
- PMID: 32376769
- PMCID: PMC8008103
- DOI: 10.1126/scitranslmed.aax4517
BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis
Abstract
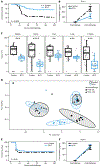
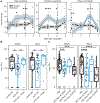
Death from sepsis in the neonatal period remains a serious threat for millions. Within 3 days of administration, bacille Calmette-Guérin (BCG) vaccination can reduce mortality from neonatal sepsis in human newborns, but the underlying mechanism for this rapid protection is unknown. We found that BCG was also protective in a mouse model of neonatal polymicrobial sepsis, where it induced granulocyte colony-stimulating factor (G-CSF) within hours of administration. This was necessary and sufficient to drive emergency granulopoiesis (EG), resulting in a marked increase in neutrophils. This increase in neutrophils was directly and quantitatively responsible for protection from sepsis. Rapid induction of EG after BCG administration also occurred in three independent cohorts of human neonates.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


References
-
- U. N. C. s. Fund, in U.N. Data, U.N., Ed. (U.N., Geneva, 2019).
-
- Shane AL, Stoll BJ, Neonatal sepsis: Progress towards improved outcomes. J. Infect 68 (Suppl. 1), S24–S32 (2014). - PubMed
-
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, Lalli M, Bhutta Z, Barros AJ, Christian P, Mathers C, Cousens SN, Every newborn: Progress, priorities, and potential beyond survival. Lancet 384, 189–205 (2014). - PubMed
-
- Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, Zaidi AK, Berkley JA, Cousens SN, Lawn JE, Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: A systematic review and meta-analysis. Lancet Infect. Dis 14, 731–741 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

