Somatic Tissue Engineering in Mouse Models Reveals an Actionable Role for WNT Pathway Alterations in Prostate Cancer Metastasis
- PMID: 32376773
- PMCID: PMC7334089
- DOI: 10.1158/2159-8290.CD-19-1242
Somatic Tissue Engineering in Mouse Models Reveals an Actionable Role for WNT Pathway Alterations in Prostate Cancer Metastasis
Abstract
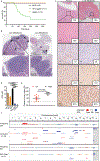
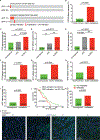
To study genetic factors influencing the progression and therapeutic responses of advanced prostate cancer, we developed a fast and flexible system that introduces genetic alterations relevant to human disease directly into the prostate glands of mice using tissue electroporation. These electroporation-based genetically engineered mouse models (EPO-GEMM) recapitulate features of traditional germline models and, by modeling genetic factors linked to late-stage human disease, can produce tumors that are metastatic and castration-resistant. A subset of tumors with Trp53 alterations acquired spontaneous WNT pathway alterations, which are also associated with metastatic prostate cancer in humans. Using the EPO-GEMM approach and an orthogonal organoid-based model, we show that WNT pathway activation drives metastatic disease that is sensitive to pharmacologic WNT pathway inhibition. Thus, by leveraging EPO-GEMMs, we reveal a functional role for WNT signaling in driving prostate cancer metastasis and validate the WNT pathway as therapeutic target in metastatic prostate cancer. SIGNIFICANCE: Our understanding of the factors driving metastatic prostate cancer is limited by the paucity of models of late-stage disease. Here, we develop EPO-GEMMs of prostate cancer and use them to identify and validate the WNT pathway as an actionable driver of aggressive metastatic disease.This article is highlighted in the In This Issue feature, p. 890.
©2020 American Association for Cancer Research.
Conflict of interest statement
CONFLICTS OF INTEREST
W.A. received an honorarium from CARET, and is a consultant for Clovis Oncology, Janssen, MORE Health, and ORIC Pharmaceuticals. C.L.S. serves on the Board of Directors of Novartis, is a co-founder of ORIC Pharmaceuticals and co-inventor of enzalutamide and apalutamide. He is a science advisor to Agios, Beigene, Blueprint, Column Group, Foghorn, Housey Pharma, Nextech, KSQ, Petra, and PMV. S.W.L. is a co-founder and scientific advisory board member of Blueprint Medicines, ORIC Pharmaceuticals, Mirimus, Inc., Faeth Therapeutics, and Geras Bio, and is an advisor to Petra Pharmaceuticals, Constellation Pharmaceuticals, and Boehringer Ingelheim. L.E.D is an advisory board member for Mirimus Inc.
Figures





References
-
- Huggins C Control of cancers of man by endocrinologic methods. Cancer Res 1957;17(5):467–72. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA193837/CA/NCI NIH HHS/United States
- P50 CA092629/CA/NCI NIH HHS/United States
- R01 CA233944/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 CA155169/CA/NCI NIH HHS/United States
- P01 CA087497/CA/NCI NIH HHS/United States
- U54 CA224079/CA/NCI NIH HHS/United States
- R01 CA183929/CA/NCI NIH HHS/United States
- T32 CA160001/CA/NCI NIH HHS/United States
- U54 OD020355/OD/NIH HHS/United States
- K99 CA241110/CA/NCI NIH HHS/United States
- R01 CA173481/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

