T cell exosome-derived miR-142-3p impairs glandular cell function in Sjögren's syndrome
- PMID: 32376798
- PMCID: PMC7253014
- DOI: 10.1172/jci.insight.133497
T cell exosome-derived miR-142-3p impairs glandular cell function in Sjögren's syndrome
Abstract
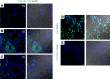
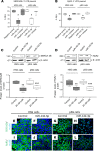
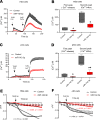
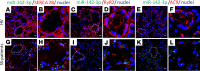
Sjögren's syndrome (SS) is a systemic autoimmune disease that mainly affects exocrine salivary and lacrimal glands. Local inflammation in the glands is thought to trigger glandular dysfunction and symptoms of dryness. However, the mechanisms underlying these processes are incompletely understood. Our work suggests T cell exosome-derived miR-142-3p as a pathogenic driver of immunopathology in SS. We first document miR-142-3p expression in the salivary glands of patients with SS, both in epithelial gland cells and within T cells of the inflammatory infiltrate, but not in healthy volunteers. Next, we show that activated T cells secreted exosomes containing miR-142-3p, which transferred into glandular cells. Finally, we uncover a functional role of miR-142-3p-containing exosomes in glandular cell dysfunction. We find that miR-142-3p targets key elements of intracellular Ca2+ signaling and cAMP production - sarco(endo)plasmic reticulum Ca2+ ATPase 2b (SERCA2B), ryanodine receptor 2 (RyR2), and adenylate cyclase 9 (AC9) - leading to restricted cAMP production, altered calcium signaling, and decreased protein production from salivary gland cells. Our work provides evidence for a functional role of the miR-142-3p in SS pathogenesis and promotes the concept that T cell activation may directly impair epithelial cell function through secretion of miRNA-containing exosomes.
Keywords: Autoimmune diseases; Autoimmunity.
Conflict of interest statement
Figures




References
-
- Brito-Zerón P, et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjögren’s syndrome at diagnosis in 8310 patients: a cross-sectional study from the Big Data Sjögren Project Consortium. Ann Rheum Dis. 2017;76(6):1042–1050. doi: 10.1136/annrheumdis-2016-209952. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

