High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi
- PMID: 32376860
- PMCID: PMC7203201
- DOI: 10.1038/s41467-020-15786-9
High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi
Abstract
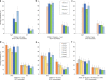
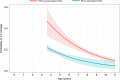
There are concerns that pneumococcal conjugate vaccines (PCVs) in sub-Saharan Africa sub-optimally interrupt Streptococcus pneumoniae vaccine-serotype (VT) carriage and transmission. Here we assess PCV carriage using rolling, prospective nasopharyngeal carriage surveys between 2015 and 2018, 3.6-7.1 years after Malawi's 2011 PCV13 introduction. Carriage decay rate is analysed using non-linear regression. Despite evidence of reduction in VT carriage over the study period, there is high persistent residual carriage. This includes among PCV-vaccinated children 3-5-year-old (16.1% relative reduction from 19.9% to 16.7%); PCV-unvaccinated children 6-8-year-old (40.5% reduction from 26.4% to 15.7%); HIV-infected adults 18-40-years-old on antiretroviral therapy (41.4% reduction from 15.2% to 8.9%). VT carriage prevalence half-life is similar among PCV-vaccinated and PCV-unvaccinated children (3.26 and 3.34 years, respectively). Compared with high-income settings, there is high residual VT carriage 3.6-7.1 years after PCV introduction. Rigorous evaluation of strategies to augment vaccine-induced control of carriage, including alternative schedules and catch-up campaigns, is required.
Conflict of interest statement
N.B-Z. reports investigator-initiated research grants from GlaxoSmithKline Biologicals and from Takeda Pharmaceuticals outside the submitted work. No other authors declare competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

