APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline
- PMID: 32376954
- PMCID: PMC7250000
- DOI: 10.1038/s41586-020-2247-3
APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline
Abstract
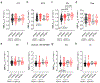
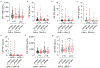
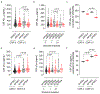
Vascular contributions to dementia and Alzheimer's disease are increasingly recognized1-6. Recent studies have suggested that breakdown of the blood-brain barrier (BBB) is an early biomarker of human cognitive dysfunction7, including the early clinical stages of Alzheimer's disease5,8-10. The E4 variant of apolipoprotein E (APOE4), the main susceptibility gene for Alzheimer's disease11-14, leads to accelerated breakdown of the BBB and degeneration of brain capillary pericytes15-19, which maintain BBB integrity20-22. It is unclear, however, whether the cerebrovascular effects of APOE4 contribute to cognitive impairment. Here we show that individuals bearing APOE4 (with the ε3/ε4 or ε4/ε4 alleles) are distinguished from those without APOE4 (ε3/ε3) by breakdown of the BBB in the hippocampus and medial temporal lobe. This finding is apparent in cognitively unimpaired APOE4 carriers and more severe in those with cognitive impairment, but is not related to amyloid-β or tau pathology measured in cerebrospinal fluid or by positron emission tomography23. High baseline levels of the BBB pericyte injury biomarker soluble PDGFRβ7,8 in the cerebrospinal fluid predicted future cognitive decline in APOE4 carriers but not in non-carriers, even after controlling for amyloid-β and tau status, and were correlated with increased activity of the BBB-degrading cyclophilin A-matrix metalloproteinase-9 pathway19 in cerebrospinal fluid. Our findings suggest that breakdown of the BBB contributes to APOE4-associated cognitive decline independently of Alzheimer's disease pathology, and might be a therapeutic target in APOE4 carriers.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures
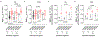




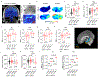

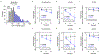

Comment in
-
Risk factor for Alzheimer's disease breaks the blood-brain barrier.Nature. 2020 May;581(7806):31-32. doi: 10.1038/d41586-020-01152-8. Nature. 2020. PMID: 32350425 Free PMC article.
-
APOE*ε4 is linked to BBB breakdown.Nat Rev Neurol. 2020 Jul;16(7):350. doi: 10.1038/s41582-020-0367-x. Nat Rev Neurol. 2020. PMID: 32393877 No abstract available.
-
Leaky memories: Impact of APOE4 on blood-brain barrier and dementia.J Cereb Blood Flow Metab. 2020 Sep;40(9):1912-1914. doi: 10.1177/0271678X20938146. Epub 2020 Jun 29. J Cereb Blood Flow Metab. 2020. PMID: 32600092 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- P30 AG066444/AG/NIA NIH HHS/United States
- R01 AG023084/AG/NIA NIH HHS/United States
- R01 AG031581/AG/NIA NIH HHS/United States
- R01 AG064228/AG/NIA NIH HHS/United States
- K01 AG062683/AG/NIA NIH HHS/United States
- R01 NS100459/NS/NINDS NIH HHS/United States
- R01 EB009352/EB/NIBIB NIH HHS/United States
- R01 AG055770/AG/NIA NIH HHS/United States
- R00 AG058780/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P01 AG052350/AG/NIA NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 AG039452/AG/NIA NIH HHS/United States
- K99 AG058780/AG/NIA NIH HHS/United States
- R01 AG060049/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- R01 NS034467/NS/NINDS NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- R01 NS090904/NS/NINDS NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- R01 AG054434/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

