Childhood vaccines and antibiotic use in low- and middle-income countries
- PMID: 32376956
- PMCID: PMC7332418
- DOI: 10.1038/s41586-020-2238-4
Childhood vaccines and antibiotic use in low- and middle-income countries
Abstract
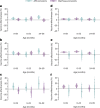
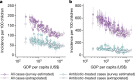
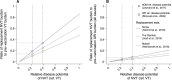
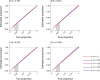
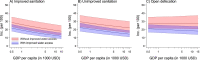
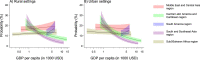
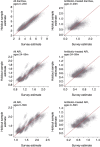
Vaccines may reduce the burden of antimicrobial resistance, in part by preventing infections for which treatment often includes the use of antibiotics1-4. However, the effects of vaccination on antibiotic consumption remain poorly understood-especially in low- and middle-income countries (LMICs), where the burden of antimicrobial resistance is greatest5. Here we show that vaccines that have recently been implemented in the World Health Organization's Expanded Programme on Immunization reduce antibiotic consumption substantially among children under five years of age in LMICs. By analysing data from large-scale studies of households, we estimate that pneumococcal conjugate vaccines and live attenuated rotavirus vaccines confer 19.7% (95% confidence interval, 3.4-43.4%) and 11.4% (4.0-18.6%) protection against antibiotic-treated episodes of acute respiratory infection and diarrhoea, respectively, in age groups that experience the greatest disease burden attributable to the vaccine-targeted pathogens6,7. Under current coverage levels, pneumococcal and rotavirus vaccines prevent 23.8 million and 13.6 million episodes of antibiotic-treated illness, respectively, among children under five years of age in LMICs each year. Direct protection resulting from the achievement of universal coverage targets for these vaccines could prevent an additional 40.0 million episodes of antibiotic-treated illness. This evidence supports the prioritization of vaccines within the global strategy to combat antimicrobial resistance8.
Conflict of interest statement
J.A.L. has received consulting fees and research grants from Pfizer, consulting fees and research grants from Merck Sharp & Dohme and research grants from the World Health Organization, all for unrelated work. N.C.L. has received personal fees from the World Health Organization, for unrelated work. All other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

