On the Possibility of Glucose Sensing Using Boronic Acid and a Luminescent Ruthenium Metal-Ligand Complex
- PMID: 32377061
- PMCID: PMC7202357
- DOI: 10.1023/A:1016800515030
On the Possibility of Glucose Sensing Using Boronic Acid and a Luminescent Ruthenium Metal-Ligand Complex
Abstract
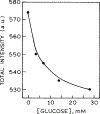
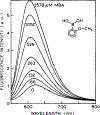
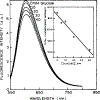
We describe a new approach to optical sensing of glucose based on the competitive interactions between a ruthenium metal ligand complex, a boronic acid derivative and glucose. The metal-ligand complex [Ru(2,2'-bipyridme)2(5,6-dihydroxy-1,10-phenanthrolme)](PF6)2 at pH 8 forms a reversible complex with 2-toluylboronic acid or 2-methoxyphenyl boronic acid. Complexation is accompanied by a several-fold increase in the luminescent intensity of the ruthenium complex. Addition of glucose results in decreased luminescent intensity, which appears to be the result of decreased binding between the metal-ligand complex and the boronic acid. Ruthenium metal-ligand complexes are convenient for optical sensing because their long luminescent decay times allow lifetime-based sensing with simple instrumentation.
Keywords: Glucose sensing; boronic acid; metal-ligand complex.
Figures



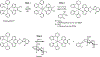
Similar articles
-
Photophysical studies of bioconjugated ruthenium metal-ligand complexes incorporated in phospholipid membrane bilayers.Inorg Chem. 2013 Oct 7;52(19):10835-45. doi: 10.1021/ic400706u. Epub 2013 Sep 24. Inorg Chem. 2013. PMID: 24063694 Free PMC article.
-
Lifetime-based sensing of glucose using energy transfer with a long lifetime donor.Anal Biochem. 1997 Jul 15;250(1):102-8. doi: 10.1006/abio.1997.2180. Anal Biochem. 1997. PMID: 9234903 Free PMC article.
-
Long-Lifetime Metal-Ligand Complexes as Luminescent Probes for DNA.J Fluoresc. 1997 Jun;7(2):107-112. doi: 10.1007/BF02760501. J Fluoresc. 1997. PMID: 31903017 Free PMC article.
-
Highly sensitive and selective phosphorescent chemosensors for hypochlorous acid based on ruthenium(II) complexes.Biosens Bioelectron. 2013 Dec 15;50:1-7. doi: 10.1016/j.bios.2013.06.005. Epub 2013 Jun 15. Biosens Bioelectron. 2013. PMID: 23827370
-
Selective sensing of saccharides using simple boronic acids and their aggregates.Chem Soc Rev. 2013 Oct 21;42(20):8032-48. doi: 10.1039/c3cs60148j. Chem Soc Rev. 2013. PMID: 23860576 Review.
Cited by
-
Multiple molecular logic gate arrays in one system of (2-(2'-pyridyl)imidazole)Ru(ii) complexes and trimeric cyclophanes in water.Chem Sci. 2022 Aug 26;13(36):10856-10867. doi: 10.1039/d2sc03617g. eCollection 2022 Sep 21. Chem Sci. 2022. PMID: 36320709 Free PMC article.
References
-
- National Institutes of Diabetes and Digestive and Kidney Disorders (June 1993). The Diabetes Control and Complications Trial, NIH Publication.
-
- McNichols RJ and Cote GL (1999) J. Biomed. Optics (in press.) - PubMed
-
- Schultz JS and Sims G (1979)Biotech. Bioeng. Symp 9, 65–71. - PubMed
-
- Schultz J, Mansouri S, and Goldstein IJ (1982) Diabetes Care Bioeng. 5(3), 245–253. - PubMed
-
- Meadows D and Schultz JS, (1988) Talanta 35(2), 145–150. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
