On the Possibility of Glucose Sensing Using Boronic Acid and a Luminescent Ruthenium Metal-Ligand Complex
- PMID: 32377061
- PMCID: PMC7202357
- DOI: 10.1023/A:1016800515030
On the Possibility of Glucose Sensing Using Boronic Acid and a Luminescent Ruthenium Metal-Ligand Complex
Abstract
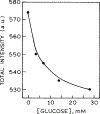
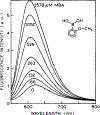
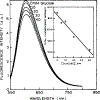
We describe a new approach to optical sensing of glucose based on the competitive interactions between a ruthenium metal ligand complex, a boronic acid derivative and glucose. The metal-ligand complex [Ru(2,2'-bipyridme)2(5,6-dihydroxy-1,10-phenanthrolme)](PF6)2 at pH 8 forms a reversible complex with 2-toluylboronic acid or 2-methoxyphenyl boronic acid. Complexation is accompanied by a several-fold increase in the luminescent intensity of the ruthenium complex. Addition of glucose results in decreased luminescent intensity, which appears to be the result of decreased binding between the metal-ligand complex and the boronic acid. Ruthenium metal-ligand complexes are convenient for optical sensing because their long luminescent decay times allow lifetime-based sensing with simple instrumentation.
Keywords: Glucose sensing; boronic acid; metal-ligand complex.
Figures



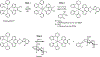
References
-
- National Institutes of Diabetes and Digestive and Kidney Disorders (June 1993). The Diabetes Control and Complications Trial, NIH Publication.
-
- McNichols RJ and Cote GL (1999) J. Biomed. Optics (in press.) - PubMed
-
- Schultz JS and Sims G (1979)Biotech. Bioeng. Symp 9, 65–71. - PubMed
-
- Schultz J, Mansouri S, and Goldstein IJ (1982) Diabetes Care Bioeng. 5(3), 245–253. - PubMed
-
- Meadows D and Schultz JS, (1988) Talanta 35(2), 145–150. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
