Nicotinamide mononucleotide attenuates glucocorticoid‑induced osteogenic inhibition by regulating the SIRT1/PGC‑1α signaling pathway
- PMID: 32377728
- PMCID: PMC7248519
- DOI: 10.3892/mmr.2020.11116
Nicotinamide mononucleotide attenuates glucocorticoid‑induced osteogenic inhibition by regulating the SIRT1/PGC‑1α signaling pathway
Abstract
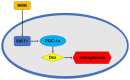
Long-term and high-dose glucocorticoid treatment is recognized as an important influencing factor for osteoporosis and osteonecrosis. Nicotinamide mononucleotide (NMN) is an intermediate of NAD+ biosynthesis, and is widely used to replenish the levels of NAD+. However, the potential role of NMN in glucocorticoid‑induced osteogenic inhibition remains to be demonstrated. In the present study, the protective effects of NMN on dexamethasone (Dex)‑induced osteogenic inhibition, and its underlying mechanisms, were investigated. Bone mesenchymal stem cells were treated with Dex, which decreased the levels of the osteogenic markers alkaline phosphatase, Runt‑related transcription factor 2 and osteocalcin. NMN treatment attenuated Dex‑induced osteogenic inhibition and promoted the expression of sirtuin 1 (SIRT1) and peroxisome proliferator‑activated receptor gamma coactivator (PGC)‑1α. SIRT1 knockdown reversed the protective effects of NMN and reduced the expression levels of PGC‑1α. Collectively, the results of the present study reveal that NMN may be a potential therapeutic target for glucocorticoid‑induced osteoporosis.
Keywords: nicotinamide mononucleotide; osteoporosis; sirtuin 1; peroxisome proliferator‑activated receptor gamma coactivator.
Figures




References
-
- Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD, Narula HS, et al. American association of clinical endocrinologists and American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016-executive summary. Endocr Pract. 2016;22:1111–1118. doi: 10.4158/EP161435.ESGL. - DOI - PubMed
-
- Yuasa M, Yamada T, Taniyama T, Masaoka T, Xuetao W, Yoshii T, Horie M, Yasuda H, Uemura T, Okawa A, Sotome S. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS One. 2015;10:e0116462. doi: 10.1371/journal.pone.0116462. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

