Phosphodiesterase 10A Inhibition Leads to Brain Region-Specific Recovery Based on Stroke Type
- PMID: 32378029
- PMCID: PMC7644574
- DOI: 10.1007/s12975-020-00819-8
Phosphodiesterase 10A Inhibition Leads to Brain Region-Specific Recovery Based on Stroke Type
Erratum in
-
Correction to: Phosphodiesterase 10A Inhibition Leads to Brain Region-Specific Recovery Based on Stroke Type.Transl Stroke Res. 2021 Aug;12(4):691. doi: 10.1007/s12975-020-00858-1. Transl Stroke Res. 2021. PMID: 33123953 Free PMC article. No abstract available.
Abstract
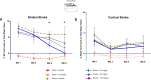
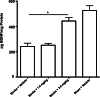
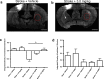
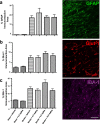
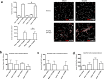
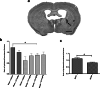
Stroke is the leading cause of adult disability. Recovery of function after stroke involves signaling events that are mediated by cAMP and cGMP pathways, such as axonal sprouting, neurogenesis, and synaptic plasticity. cAMP and cGMP are degraded by phosphodiesterases (PDEs), which are differentially expressed in brain regions. PDE10A is highly expressed in the basal ganglia/striatum. We tested a novel PDE10A inhibitor (TAK-063) for its effects on functional recovery. Stroke was produced in mice in the cortex or the striatum. Behavioral recovery was measured to 9 weeks. Tissue outcome measures included analysis of growth factor levels, angiogenesis, neurogenesis, gliogenesis, and inflammation. TAK-063 improved motor recovery after striatal stroke in a dose-related manner, but not in cortical stroke. Recovery of motor function correlated with increases in striatal brain-derived neurotrophic factor. TAK-063 treatment also increased motor system axonal connections. Stroke affects distinct brain regions, with each comprising different cellular and molecular elements. Inhibition of PDE10A improved recovery of function after striatal but not cortical stroke, consistent with its brain localization. This experiment is the first demonstration of brain region-specific enhanced functional recovery after stroke, and indicates that differential molecular signaling between brain regions can be exploited to improve recovery based on stroke subtype.
Keywords: Angiogenesis; Axonal sprouting; BDNF; Repair; Striatum.
Conflict of interest statement
S Thomas Carmichael has received a research grant from Takeda Pharmaceutical Company Limited. This funder had no role in data analysis or interpretation. Kazunori Suzuki and Haruhide Kimura are employed by Takeda Pharmaceutical Company Limited. As experts in TAK-063, these two authors had a role in experimental design but no role in data analysis or interpretation.
Figures
References
-
- Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, Pomeroy VM, Langhorne P, Cochrane Stroke Group Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev. 2014;4:CD001920. doi: 10.1002/14651858.CD001920.pub3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

