Healthcare providers' adherence to breast cancer guidelines in Europe: a systematic literature review
- PMID: 32378052
- PMCID: PMC7220981
- DOI: 10.1007/s10549-020-05657-8
Healthcare providers' adherence to breast cancer guidelines in Europe: a systematic literature review
Abstract
Purpose: Clinical guidelines' (CGs) adherence supports high-quality care. However, healthcare providers do not always comply with CGs recommendations. This systematic literature review aims to assess the extent of healthcare providers' adherence to breast cancer CGs in Europe and to identify the factors that impact on healthcare providers' adherence.
Methods: We searched for systematic reviews and quantitative or qualitative primary studies in MEDLINE and Embase up to May 2019. The eligibility assessment, data extraction, and risk of bias assessment were conducted by one author and cross-checked by a second author. We conducted a narrative synthesis attending to the modality of the healthcare process, methods to measure adherence, the scope of the CGs, and population characteristics.
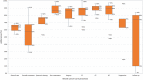
Results: Out of 8137 references, we included 41 primary studies conducted in eight European countries. Most followed a retrospective cohort design (19/41; 46%) and were at low or moderate risk of bias. Adherence for overall breast cancer care process (from diagnosis to follow-up) ranged from 54 to 69%; for overall treatment process [including surgery, chemotherapy (CT), endocrine therapy (ET), and radiotherapy (RT)] the median adherence was 57.5% (interquartile range (IQR) 38.8-67.3%), while for systemic therapy (CT and ET) it was 76% (IQR 68-77%). The median adherence for the processes assessed individually was higher, ranging from 74% (IQR 10-80%), for the follow-up, to 90% (IQR 87-92.5%) for ET. Internal factors that potentially impact on healthcare providers' adherence were their perceptions, preferences, lack of knowledge, or intentional decisions.
Conclusions: A substantial proportion of breast cancer patients are not receiving CGs-recommended care. Healthcare providers' adherence to breast cancer CGs in Europe has room for improvement in almost all care processes. CGs development and implementation processes should address the main factors that influence healthcare providers' adherence, especially patient-related ones.
Registration: PROSPERO (CRD42018092884).
Keywords: Breast neoplasms; Evidence-based medicine; Guideline adherence; Guidelines as topic; Systematic review.
Conflict of interest statement
Zuleika Saz Parkinson, Luciana Neamtiu, and Elena Parmelli are current employees of the Joint Research Centre, European Commission; Ignacio Ricci-Cabello, Ena Niño de Guzmán, Javier Pérez-Bracchiglione, Yang Song, Montserrat Rabassa, Iván Solà, David Rigau, Carlos Canelo-Aybar, and Pablo Alonso-Coello are employees of the Iberoamerican Cochrane Center.
Figures
References
-
- Institute of Medicine (US) Committee on Clinical Practice Guidelines. Field MJ, Lohr KN. Guidelines for clinical practice: from development to use. Washington (DC): National Academies Press (US); 1992. - PubMed
-
- Clinical Practice Guidelines We Can Trust (2011) National Academy Press (US), Washington DC. https://www.ncbi.nlm.nih.gov/books/NBK209546/. Accessed 31 July 2019
-
- Gurses AP, Marsteller JA, Ozok AA, Xiao Y, Owens S, Pronovost PJ. Using an interdisciplinary approach to identify factors that affect clinicians' compliance with evidence-based guidelines. Crit Care Med. 2010;38(8 Suppl):S282–S291. - PubMed
-
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. - PubMed
-
- Levit L, Balogh E, Nass S, Ganz PA. Committee on improving the quality of cancer care: addressing the challenges of an aging population BoHCS, institute of medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington (DC): National Academies Press (US); 2013. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

