Nanobiotechnological modules as molecular target tracker for the treatment and prevention of malaria: options and opportunity
- PMID: 32378173
- PMCID: PMC7223109
- DOI: 10.1007/s13346-020-00770-z
Nanobiotechnological modules as molecular target tracker for the treatment and prevention of malaria: options and opportunity
Abstract
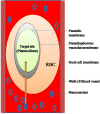
Malaria is one of the major infectious diseases that remains a constant challenge to human being mainly due to the emergence of drug-resistant strains of parasite and also the availability of drugs, which are non-specific for their pharmacodynamic activity and known to be associated with multiple side effects. The disease has acquired endemic proportions in tropical countries where the hygienic conditions are not satisfactory while the environmental conditions favor the proliferation of parasite and its transmission, particularly through the female anopheles. It is obvious that to square up the problems, there is a need for designing and development of more effective drugs, which can combat the drug-resistant strains of the parasite. Molecular biology of the parasite and its homing into host cellular tropics provide multiple drug targets that could judiciously be considered for engineering and designing of new generation antimalarial drugs and also drug delivery systems. Though the recent reports document that against malaria parasite the vaccine could be developed, nevertheless, due to smart mutational change overs by the parasite, it is able to bypass the immune surveillance. The developed vaccine therefore failed to assure absolute protection against the malarial infection. In the conventional mode of treatment antimalarial drugs, the dose and dosage regimen that is followed at large crops up the contraindicative manifestations, and hence compromising the effective treatment. The emerging trends and new updates in contemporary biological sciences, material sciences, and drug delivery domain have enabled us with the availability of a multitude of mode and modules which could plunge upon the nanotechnology in particular to treat this challenging infection. The nanotechnology-based option may be tuned or customized as per the requirements to mark and target i.e. the infected RBCs, for targeted drug delivery. Graphical abstract.
Keywords: Drug resistance; Malaria; Nanobiotechnology; Plasmodium; RBC; Vaccine.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
-
- Margaret A, Phillips JNB, Christine Manyando, van Huijsduijnen Rob Hooft, Van Voorhis Wesley C, Timothy N. C. Wells Malaria. NATURE REVIEWS | DISEASE PRIMERS 2017;3. - PubMed
-
- Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004;3(6):509–520. - PubMed
-
- Baruah UK, Gowthamarajan K, Vanka R, Karri VVSR, Selvaraj K, Jojo GM. Malaria treatment using novel nano-based drug delivery systems. J Drug Target. 2017;25(7):567–581. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

