Dysfunction and toxicity of damaged proteins in the etiology of aging and age-related degenerative and malignant diseases
- PMID: 32378382
- PMCID: PMC7230427
- DOI: 10.3325/cmj.2020.61.159
Dysfunction and toxicity of damaged proteins in the etiology of aging and age-related degenerative and malignant diseases
Abstract
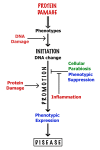
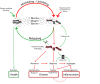
Health can be defined as a harmony, or homeostasis, of the activities of thousands of different proteins, whereas aging and diseases result from their disharmony manifested at the levels of cells and tissues. Such disharmony is caused primarily by dysfunction and toxicity of misfolded proteins damaged by oxidation. This is an overview of key data that inspired new concepts allowing interpretation and integration of the scientific literature on aging and age-related diseases. These concepts suggest strategies for prevention and attenuation of age-related degenerative and malignant diseases mimicking the life of super-centenarians.
Figures
References
-
- Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987;262:5488–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical