The Self-Assembly of a Cyclometalated Palladium Photosensitizer into Protein-Stabilized Nanorods Triggers Drug Uptake In Vitro and In Vivo
- PMID: 32378894
- PMCID: PMC7291344
- DOI: 10.1021/jacs.0c01369
The Self-Assembly of a Cyclometalated Palladium Photosensitizer into Protein-Stabilized Nanorods Triggers Drug Uptake In Vitro and In Vivo
Abstract
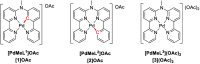
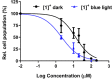
Enhanced passive diffusion is usually considered to be the primary cause of the enhanced cellular uptake of cyclometalated drugs because cyclometalation lowers the charge of a metal complex and increases its lipophilicity. However, in this work, monocationic cyclometalated palladium complexes [1]OAc (N^N^C^N) and [2]OAc (N^N^N^C) were found to self-assemble, in aqueous solutions, into soluble supramolecular nanorods, while their tetrapyridyl bicationic analogue [3](OAc)2 (N^N^N^N) dissolved as isolated molecules. These nanorods formed via metallophilic Pd···Pd interaction and π-π stacking and were stabilized in the cell medium by serum proteins, in the absence of which the nanorods precipitated. In cell cultures, these protein-stabilized self-assembled nanorods were responsible for the improved cellular uptake of the cyclometalated compounds, which took place via endocytosis (i.e., an active uptake pathway). In addition to triggering self-assembly, cyclometalation in [1]OAc also led to dramatically enhanced photodynamic properties under blue light irradiation. These combined penetration and photodynamic properties were observed in multicellular tumor spheroids and in a mice tumor xenograft, demonstrating that protein-stabilized nanoaggregation of cyclometalated drugs such as [1]OAc also allows efficient cellular uptake in 3D tumor models. Overall, serum proteins appear to be a major element in drug design because they strongly influence the size and bioavailability of supramolecular drug aggregates and hence their efficacy in vitro and in vivo.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
The two isomers of a cyclometallated palladium sensitizer show different photodynamic properties in cancer cells.Chem Commun (Camb). 2019 Apr 16;55(32):4695-4698. doi: 10.1039/c8cc10134e. Chem Commun (Camb). 2019. PMID: 30942201
-
Zinc phthalocyanines attached to gold nanorods for simultaneous hyperthermic and photodynamic therapies against melanoma in vitro.J Photochem Photobiol B. 2017 Aug;173:181-186. doi: 10.1016/j.jphotobiol.2017.05.037. Epub 2017 May 30. J Photochem Photobiol B. 2017. PMID: 28595072 Free PMC article.
-
Guanidine-modified cyclometalated iridium(III) complexes for mitochondria-targeted imaging and photodynamic therapy.Eur J Med Chem. 2019 Oct 1;179:26-37. doi: 10.1016/j.ejmech.2019.06.045. Epub 2019 Jun 18. Eur J Med Chem. 2019. PMID: 31233920
-
A palladium label to monitor nanoparticle-assisted drug delivery of a photosensitizer into tumor spheroids by elemental bioimaging.Metallomics. 2014 Jan;6(1):77-81. doi: 10.1039/c3mt00223c. Metallomics. 2014. PMID: 24311052
-
Photoresponsive ruthenium-containing polymers: potential polymeric metallodrugs for anticancer phototherapy.Dalton Trans. 2018 Jan 2;47(2):283-286. doi: 10.1039/c7dt03390g. Dalton Trans. 2018. PMID: 29177301 Review.
Cited by
-
Rollover Cyclometalation vs Nitrogen Coordination in Tetrapyridyl Anticancer Gold(III) Complexes: Effect on Protein Interaction and Toxicity.JACS Au. 2021 Apr 26;1(4):380-395. doi: 10.1021/jacsau.0c00104. Epub 2021 Mar 16. JACS Au. 2021. PMID: 34056633 Free PMC article.
-
Metal-Coordinated Supramolecular Self-Assemblies for Cancer Theranostics.Adv Sci (Weinh). 2021 Aug;8(16):e2101101. doi: 10.1002/advs.202101101. Epub 2021 Jun 18. Adv Sci (Weinh). 2021. PMID: 34145984 Free PMC article. Review.
-
In vivo metallophilic self-assembly of a light-activated anticancer drug.Nat Chem. 2023 Jul;15(7):980-987. doi: 10.1038/s41557-023-01199-w. Epub 2023 May 11. Nat Chem. 2023. PMID: 37169984 Free PMC article.
-
Fine-Feature Modifications to Strained Ruthenium Complexes Radically Alter Their Hypoxic Anticancer Activity†.Photochem Photobiol. 2022 Jan;98(1):73-84. doi: 10.1111/php.13395. Epub 2021 Mar 28. Photochem Photobiol. 2022. PMID: 33559191 Free PMC article.
-
Exploring the antitumor effects of Pd(II) complexes with nitrogen donor ligands towards breast carcinoma.Biometals. 2025 Aug;38(4):1235-1254. doi: 10.1007/s10534-025-00702-9. Epub 2025 Jun 5. Biometals. 2025. PMID: 40471376
References
-
- Štarha P.; Vančo J.; Trávníček Z. Platinum iodido complexes: A comprehensive overview of anticancer activity and mechanisms of action. Coord. Chem. Rev. 2019, 380, 103–135. 10.1016/j.ccr.2018.09.017. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

