Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age
- PMID: 32379417
- PMCID: PMC7241737
- DOI: 10.1021/acschemneuro.0c00210
Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age
Abstract
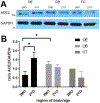
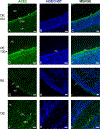
The COVID-19 pandemic revealed that there is a loss of smell in many patients, including in infected but otherwise asymptomatic individuals. The underlying mechanisms for the olfactory symptoms are unclear. Using a mouse model, we determined whether cells in the olfactory epithelium express the obligatory receptors for entry of the SARS-CoV-2 virus by using RNAseq, RT-PCR, in situ hybridization, Western blot, and immunocytochemistry. We show that the cell surface protein ACE2 and the protease TMPRSS2 are expressed in sustentacular cells of the olfactory epithelium but not, or much less, in most olfactory receptor neurons. These data suggest that sustentacular cells are involved in SARS-CoV-2 virus entry and impairment of the sense of smell in COVID-19 patients. We also show that expression of the entry proteins increases in animals of old age. This may explain, if true also in humans, why individuals of older age are more susceptible to the SARS-CoV-2 infection.
Keywords: ACE2 expression; COVID-19; SARS-CoV-2; aging; anosmia; olfactory epithelium.
Figures



Comment in
-
Taste and Smell Impairment in SARS-CoV-2 Recovers Early and Spontaneously: Experimental Data Strongly Linked to Clinical Data.ACS Chem Neurosci. 2020 Jul 15;11(14):2031-2033. doi: 10.1021/acschemneuro.0c00296. Epub 2020 Jul 1. ACS Chem Neurosci. 2020. PMID: 32539346
References
-
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study [Apr 6]. Eur Arch Otorhinolaryngol 2020;10.1007/s00405-020-05965-1. doi: 10.1007/s00405-020-05965-1 [Epub ahead of print] - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

