Evaluation of intercellular communication between breast cancer cells and adipose-derived stem cells via passive diffusion in a two-layer microfluidic device
- PMID: 32379852
- PMCID: PMC7331673
- DOI: 10.1039/d0lc00142b
Evaluation of intercellular communication between breast cancer cells and adipose-derived stem cells via passive diffusion in a two-layer microfluidic device
Abstract
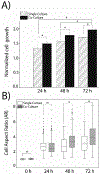
Breast cancer tumorigenesis and response to therapy is regulated by cancer cell interactions with the tumor microenvironment (TME). Breast cancer signaling to the surrounding TME results in a heterogeneous and diverse tumor microenvironment, which includes the production of cancer-associated fibroblasts, macrophages, adipocytes, and stem cells. The secretory profile of these cancer-associated cell types results in elevated chemokines and growth factors that promote cell survival and proliferation within the tumor. Current co-culture approaches mostly rely on transwell chambers to study intercellular signaling between adipose-derived stem cells (ASCs) and cancer cells; however, these methods are limited to endpoint measurements and lack dynamic control. In this study, a 4-channel, "flow-free" microfluidic device was developed to co-culture triple-negative MDA-MB-231 breast cancer cells and ASCs to study intercellular communication between two distinct cell types found in the TME. The device consists of two layers: a top PDMS layer with four imprinted channels coupled with a bottom agarose slab enclosed in a Plexiglas chamber. For dynamic co-culture, the device geometry contained two centered, flow-free channels, which were supplied with media from two outer flow channels via orthogonal diffusion through the agarose. Continuous fresh media was provided to the cell culture channel via passive diffusion without creating any shearing effect on the cells. The device geometry also allowed for the passive diffusion of cytokines and growth factors between the two cell types cultured in parallel channels to initiate cell-to-cell crosstalk. The device was used to show that MDA-MB-231 cells co-cultured with ASCs exhibited enhanced growth, a more aggressive morphology, and polarization toward the ASCs. The MDA-MB-231 cells were found to exhibit a greater degree of resistance to the drug paclitaxel when co-cultured with ASCs when compared to single culture studies. This microfluidic device is an ideal platform to study intercellular communication for many types of cells during co-culture experiments and allows for new investigations into stromal cell-mediated drug resistance in the tumor microenvironment.
Conflict of interest statement
Conflicts of interest
“There are no conflicts to declare”.
Figures





References
-
- Hanahan D and Weinberg RA, Cell, 2011, 144, 646–674. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

