Organophotoredox Hydrodefluorination of Trifluoromethylarenes with Translational Applicability to Drug Discovery
- PMID: 32379965
- PMCID: PMC7304874
- DOI: 10.1021/jacs.0c03881
Organophotoredox Hydrodefluorination of Trifluoromethylarenes with Translational Applicability to Drug Discovery
Abstract
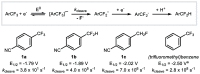
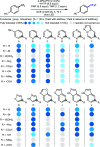
Molecular editing such as insertion, deletion, and single atom exchange in highly functionalized compounds is an aspirational goal for all chemists. Here, we disclose a photoredox protocol for the replacement of a single fluorine atom with hydrogen in electron-deficient trifluoromethylarenes including complex drug molecules. A robustness screening experiment shows that this reductive defluorination tolerates a range of functional groups and heterocycles commonly found in bioactive molecules. Preliminary studies allude to a catalytic cycle whereby the excited state of the organophotocatalyst is reductively quenched by the hydrogen atom donor, and returned in its original oxidation state by the trifluoromethylarene.
Conflict of interest statement
The authors declare the following competing financial interest(s): T.K. and C.W.A. are employees of Pfizer Inc.; C.F.M. and A.A.T. are employees of Janssen; C.G. is an employee of UCB Biopharma Sprl.
Figures




References
-
- Müller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. - DOI - PubMed
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. - DOI - PubMed
- Meanwell N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. - DOI - PubMed
- Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. - DOI - PubMed
- Clayden J. Fluorinated compounds present opportunities for drug discovery. Nature 2019, 573, 37–38. 10.1038/d41586-019-02611-7. - DOI - PubMed
-
- Sessler C. D.; Rahm M.; Becker S.; Goldberg J. M.; Wang F.; Lippard S. J. CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc. 2017, 139, 9325–9332. 10.1021/jacs.7b04457. - DOI - PMC - PubMed
- Yerien D. E.; Barata-Vallejo S.; Postigo A. Difluoromethylation reactions of organic compounds. Chem. - Eur. J. 2017, 23, 14676–14701. 10.1002/chem.201702311. - DOI - PubMed
- Rong J.; Ni C.; Hu J. Metal-Catalyzed Direct Difluoromethylation Reactions. Asian J. Org. Chem. 2017, 6, 139–152. 10.1002/ajoc.201600509. - DOI
- Lu Y.; Liu C.; Chen Q.-Y. Recent Advances in Difluoromethylation Reaction. Curr. Org. Chem. 2015, 19, 1638–1650. 10.2174/1385272819666150615235605. - DOI
-
-
For seminal examples on C(sp3)–F and C(sp2)–F functionalization, see:
- Senaweera S. M.; Weaver J. D. Selective Perfluoro- and Polyfluoroarylation of Meldrum’s Acid. J. Org. Chem. 2014, 79, 10466–10476. 10.1021/jo502075p. - DOI - PubMed
- Khaled M. B.; El Mokadem R. K.; Weaver J. D. III Hydrogen bond directed photocatalytic hydrodefluorination: overcoming electronic control. J. Am. Chem. Soc. 2017, 139, 13092–13101. 10.1021/jacs.7b06847. - DOI - PMC - PubMed
- Xu L.; Zhang Q.; Xie Q.; Huang B.; Dai J. J.; Xu J.; Xu H. J. Pd-catalyzed defluorination/arylation of α-trifluoromethyl ketones via consecutive β-F elimination and C-F bond activation. Chem. Commun. 2018, 54, 4406–4409. 10.1039/C8CC01568F. - DOI - PubMed
- Nishimine T.; Taira H.; Tokunaga E.; Shiro M.; Shibata N. Enantioselective Trichloromethylation of MBH-Fluorides with Chloroform Based on Silicon-assisted C-F Activation and Carbanion Exchange Induced by a Ruppert-Prakash Reagent. Angew. Chem., Int. Ed. 2016, 55, 359–363. 10.1002/anie.201508574. - DOI - PubMed
- Pigeon X.; Bergeron M.; Barabé F.; Dubé P.; Frost H. N.; Paquin J. F. Activation of Allylic C-F bonds: Palladium-Catalyzed Allylic Amination of 3,3-Difluoropropenes. Angew. Chem., Int. Ed. 2010, 49, 1123–1127. 10.1002/anie.200904747. - DOI - PubMed
- Hamel J. D.; Paquin J. F. Activation of C-F bonds α to C-C multiple bonds. Chem. Commun. 2018, 54, 10224–10239. 10.1039/C8CC05108A. - DOI - PubMed
- Paquin J. F. Synthesis of Monofluoroalkenes via the Activation of Allylic C-F Bonds: A Novel Route to β-Aminofluoroalkenes Using Pd-Catalyzed Allylic Amination Reactions of 3,3-Difluoropropenes. Synlett 2011, 2011, 289–293. 10.1055/s-0030-1259333. - DOI
- Haufe G.; Suzuki S.; Yasui H.; Terada C.; Kitayama T.; Shiro M.; Shibata N. C-F Bond Activation of Unactivated Aliphatic Fluorides: Synthesis of Fluoromethyl-3,5-diaryl-2-oxazolidinones by Desymmetrization of 2-Aryl-1,3-difluoropropan-2-ols. Angew. Chem., Int. Ed. 2012, 51, 12275–12279. 10.1002/anie.201207304. - DOI - PubMed
- Hazari A.; Gouverneur V.; Brown J. M. Palladium-Catalyzed Substitution of Allylic Fluorides. Angew. Chem., Int. Ed. 2009, 48, 1296–1299. 10.1002/anie.200804310. - DOI - PubMed
- Blessley G.; Holden P.; Walker M.; Brown J. M.; Gouverneur V. Palladium-Catalyzed Substitution and Cross-Coupling of Benzylic Fluorides. Org. Lett. 2012, 14, 2754–2757. 10.1021/ol300977f. - DOI - PubMed
-
For selected reviews on C–F bond activation, see:
- Amii H.; Uneyama K. C-F bond activation in organic synthesis. Chem. Rev. 2009, 109, 2119–2183. 10.1021/cr800388c. - DOI - PubMed
- Ahrens T.; Kohlmann J.; Ahrens M.; Braun T. Functionalization of Fluorinated Molecules by Transition-Metal-Mediated C-F Bond Activation to Access Fluorinated Building Blocks. Chem. Rev. 2015, 115, 931–972. 10.1021/cr500257c. - DOI - PubMed
- Senaweera S. M.; Singh A.; Weaver J. D. Photocatalytic Hydrodefluorination: Facile Access to Partially Fluorinated Aromatics. J. Am. Chem. Soc. 2014, 136, 3002–3005. 10.1021/ja500031m. - DOI - PubMed
-
-
- Andrieux C. P.; Combellas C.; Kanoufi F.; Savéant J.; Thiébault A. Dynamics of Bond Breaking in Ion Radicals. Mechanisms and Reactivity in the Reductive Cleavage of Carbon-Fluorine Bonds of Fluoromethylarenes. J. Am. Chem. Soc. 1997, 119, 9527–9540. 10.1021/ja971094o. - DOI
- Clavel P.; Lessene G.; Biran C.; Bordeau M.; Roques N.; Trévin S.; Montauzon D. D. Selective electrochemical synthesis and reactivity of functional benzylic fluorosilylsynthons. J. Fluorine Chem. 2001, 107, 301–310. 10.1016/S0022-1139(00)00373-0. - DOI
-
- Ferraris D.; Cox C.; Anand R.; Lectka T. C-F Bond Activation by Aryl Carbocations: Conclusive Intramolecular Fluoride Shifts between Carbon Atoms in Solution and the First Examples of Intermolecular Fluoride Ion Abstractions. J. Am. Chem. Soc. 1997, 119, 4319–4320. 10.1021/ja963090+. - DOI
- Yoshida S.; Shimomori K.; Kim Y.; Hosoya T. Single C-F Bond Cleavage of Trifluoromethylarenes with an ortho-Silyl Group. Angew. Chem., Int. Ed. 2016, 55, 10406–10409. 10.1002/anie.201604776. - DOI - PubMed
- Mallov I.; Ruddy A. J.; Zhu H.; Grimme S.; Stephan D. W. C-F Bond Activation by Silylium Cation/Phosphine Frustrated Lewis Pairs: Mono-Hydrodefluorination of PhCF3, PhCF2H and Ph2CF2. Chem. - Eur. J. 2017, 23, 17692–17696. 10.1002/chem.201705276. - DOI - PubMed
- Mandal D.; Gupta R.; Jaiswal A. K.; Young R. D. Frustrated Lewis-Pair-Meditated Selective Single Fluoride Substitution in Trifluoromethyl Groups. J. Am. Chem. Soc. 2020, 142, 2572–2578. 10.1021/jacs.9b12167. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

