HBV replication inhibitors
- PMID: 32380149
- PMCID: PMC7293572
- DOI: 10.1016/j.antiviral.2020.104815
HBV replication inhibitors
Abstract
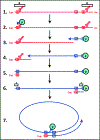
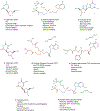
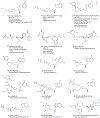
Chronic Hepatitis B Virus infections afflict >250 million people and kill nearly 1 million annually. Current non-curative therapies are dominated by nucleos(t)ide analogs (NAs) that profoundly but incompletely suppress DNA synthesis by the viral reverse transcriptase. Residual HBV replication during NA therapy contributes to maintenance of the critical nuclear reservoir of the HBV genome, the covalently-closed circular DNA, and to ongoing infection of naive cells. Identification of next-generation NAs with improved efficacy and safety profiles, often through novel prodrug approaches, is the primary thrust of ongoing efforts to improve HBV replication inhibitors. Inhibitors of the HBV ribonuclease H, the other viral enzymatic activity essential for viral genomic replication, are in preclinical development. The complexity of HBV's reverse transcription pathway offers many other potential targets. HBV's protein-priming of reverse transcription has been briefly explored as a potential target, as have the host chaperones necessary for function of the HBV reverse transcriptase. Improved inhibitors of HBV reverse transcription would reduce HBV's replication-dependent persistence mechanisms and are therefore expected to become a backbone of future curative combination anti-HBV therapies.
Keywords: Chronic hepatitis B; Hepatitis B virus; Nucleos(t)ide analogs; Reverse transcriptase inhibitors; Ribonuclease H inhibitors.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
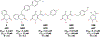
References
-
- Agarwal K, Fung SK, Nguyen TT, Cheng W, Sicard E, Ryder SD, Flaherty JF, Lawson E, Zhao S, Subramanian GM, McHutchison JG, Gane EJ, Foster GR, 2015. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J. Hepatol 62, 533–540. - PubMed
-
- Ahn SH, Kim W, Jung YK, Yang JM, Jang JY, Kweon YO, Um SH, Sohn JH, Lee JW, Park SJ, Lee BS, Kim JH, Kim HS, Yoon SK, Kim MY, Yim HJ, Lee KS, Lim YS, Lee WS, Park NH, Jin SY, Kim K-H, Choi W, Han K-H, 2019. Efficacy and safety of besifovir dipivoxyl maleate compared with tenofovir disoproxil fumarate in treatment of chronic hepatitis B virus infection. Clin. Gastroenterol. Hepatol 17, 1850–1859. - PubMed
-
- Allweiss L, Dandri M, 2016. Experimental in vitro and in vivo models for the study of human hepatitis B virus infection. J. Hepatol 64, S17–31. - PubMed
-
- Alter H, Block T, Brown N, Brownstein A, Brosgart C, Chang KM, Chen PJ, Chisari FV, Cohen C, El-Serag H, Feld J, Gish R, Glenn J, Greten T, Guo H, Guo JT, Hoshida Y, Hu J, Kowdley KV, Li W, Liang J, Locarnini S, Lok AS, Mason W, McMahon B, Mehta A, Perrillo R, Revill P, Rice CM, Rinaudo J, Schinazi R, Seeger C, Shetty K, Tavis J, Zoulim F, 2017. A research agenda for curing chronic hepatitis B virus infection. Hepatology 67, 1127–1131. - PMC - PubMed
-
- Anderson DL, 2009. Clevudine for hepatitis B. Drugs Today (Barc) 45, 331–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

