Pilot Randomized Trial of a Transdisciplinary Geriatric and Palliative Care Intervention for Older Adults With Cancer
- PMID: 32380460
- PMCID: PMC7851750
- DOI: 10.6004/jnccn.2019.7386
Pilot Randomized Trial of a Transdisciplinary Geriatric and Palliative Care Intervention for Older Adults With Cancer
Abstract
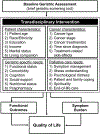
Background: Oncologists often struggle with managing the unique care needs of older adults with cancer. This study sought to determine the feasibility of delivering a transdisciplinary intervention targeting the geriatric-specific (physical function and comorbidity) and palliative care (symptoms and prognostic understanding) needs of older adults with advanced cancer.
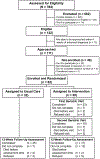
Methods: Patients aged ≥65 years with incurable gastrointestinal or lung cancer were randomly assigned to a transdisciplinary intervention or usual care. Those in the intervention arm received 2 visits with a geriatrician, who addressed patients' palliative care needs and conducted a geriatric assessment. We predefined the intervention as feasible if >70% of eligible patients enrolled in the study and >75% of eligible patients completed study visits and surveys. At baseline and week 12, we assessed patients' quality of life (QoL), symptoms, and communication confidence. We calculated mean change scores in outcomes and estimated intervention effect sizes (ES; Cohen's d) for changes from baseline to week 12, with 0.2 indicating a small effect, 0.5 a medium effect, and 0.8 a large effect.
Results: From February 2017 through June 2018, we randomized 62 patients (55.9% enrollment rate [most common reason for refusal was feeling too ill]; median age, 72.3 years; cancer types: 56.5% gastrointestinal, 43.5% lung). Among intervention patients, 82.1% attended the first visit and 79.6% attended both. Overall, 89.7% completed all study surveys. Compared with usual care, intervention patients had less QoL decrement (-0.77 vs -3.84; ES = 0.21), reduced number of moderate/severe symptoms (-0.69 vs +1.04; ES = 0.58), and improved communication confidence (+1.06 vs -0.80; ES = 0.38).
Conclusions: In this pilot trial, enrollment exceeded 55%, and >75% of enrollees completed all study visits and surveys. The transdisciplinary intervention targeting older patients' unique care needs showed encouraging ES estimates for enhancing patients' QoL, symptom burden, and communication confidence.
Figures
References
-
- Yancik R Population aging and cancer: a cross-national concern. Cancer J. 2005;11: 437–441. - PubMed
-
- Patel SS, Nelson R, Sanchez J, et al. Elderly patients with colon cancer have unique tumor characteristics and poor survival. Cancer. 2013;119: 739–747. - PubMed
-
- Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24: 2368–2375. - PubMed
-
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345: 1091–1097. - PubMed
-
- Mor V, Allen S, Malin M. The psychosocial impact of cancer on older versus younger patients and their families. Cancer. 1994;74: 2118–2127. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical