Potential Roles and Functions of Listerial Virulence Factors during Brain Entry
- PMID: 32380697
- PMCID: PMC7291126
- DOI: 10.3390/toxins12050297
Potential Roles and Functions of Listerial Virulence Factors during Brain Entry
Abstract
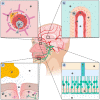
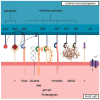
Although it rarely induces disease in humans, Listeria monocytogenes (Lm) is important due to the frequency of serious pathological conditions-such as sepsis and meningitis-it causes in those few people that do get infected. Virulence factors (VF) of Lm-especially those involved in the passage through multiple cellular barriers of the body, including internalin (Inl) family members and listeriolysin O (LLO)-have been investigated both in vitro and in vivo, but the majority of work was focused on the mechanisms utilized during penetration of the gut and fetoplacental barriers. The role of listerial VF during entry into other organs remain as only partially solved puzzles. Here, we review the current knowledge on the entry of Lm into one of its more significant destinations, the brain, with a specific focus on the role of various VF in cellular adhesion and invasion.
Keywords: Listeria monocytogenes; autolysin; brain invasion; internalin; listeriolysin; virulence factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Maiden M.C., Bygraves J.A., Feil E., Morelli G., Russell J.E., Urwin R., Zhang Q., Zhou J., Zurth K., Caugant D.A., et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad Sci. USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

