Revisiting TNF Receptor-Associated Periodic Syndrome (TRAPS): Current Perspectives
- PMID: 32380704
- PMCID: PMC7246474
- DOI: 10.3390/ijms21093263
Revisiting TNF Receptor-Associated Periodic Syndrome (TRAPS): Current Perspectives
Abstract
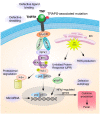
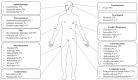
Tumor necrosis factor receptor-associated periodic syndrome (TRAPS) is an autosomal dominant autoinflammatory syndrome characterized by prolonged and recurrent episodes of fever, abdominal and/or chest pain, arthralgia, myalgia, and erythematous rash. TRAPS is associated with heterozygous variants in the TNFRSF1A gene, which encodes the TNFR1 (tumor necrosis factor receptor 1) receptor. Disease-causing variants are found exclusively in the extracellular domain of TNFR1 and affect receptor structure and binding to the TNF ligand. The precise mechanism of the disease is still unclear, but it is thought that intracellular accumulation of misfolded mutant protein leads to endoplasmic reticulum stress and enhanced inflammatory responses through constitutive activation of various immune pathways. Other possible mechanisms contributing to the disease pathogenesis include defective receptor shedding, TNF-induced cell death, production of reactive oxygen species, and autophagy impairment. Patients' leucocytes are hyperresponsive to stimulation and produce elevated levels of proinflammatory cytokines. Systemic autoimmune (AA) amyloidosis is an important cause of morbidity and mortality in TRAPS. Over the last two decades, new therapies have changed the progression and outcome of the disease. In this review, we summarize clinical data from 209 patients with validated pathogenic variants reported in the literature and discuss TRAPS diagnosis, pathogenesis, and treatment options.
Keywords: AA amyloidosis; IL-1inhibitors; TNF inhibitors; TNFR1; autoinflammatory disorders; misfolding disease; tumor necrosis factor receptor-associated periodic syndrome (TRAPS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McDermott M.F., Aksentijevich I., Galon J., McDermott E.M., Ogunkolade B.W., Centola M., Mansfield E., Gadina M., Karenko L., Pettersson T., et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/S0092-8674(00)80721-7. - DOI - PubMed
-
- Williamson L.M., Hull D., Mehta R., Reeves W.G., Robinson B.H., Toghill P.J. Familial Hibernian fever. QJM Int. J. Med. 1982;51:469–480. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

