Aberrant Mitochondrial Morphology and Function in the BTBR Mouse Model of Autism Is Improved by Two Weeks of Ketogenic Diet
- PMID: 32380723
- PMCID: PMC7246481
- DOI: 10.3390/ijms21093266
Aberrant Mitochondrial Morphology and Function in the BTBR Mouse Model of Autism Is Improved by Two Weeks of Ketogenic Diet
Abstract
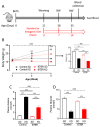
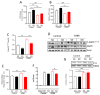
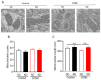
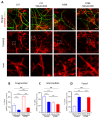
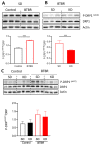
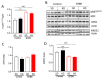
Autism spectrum disorder (ASD) is a highly prevalent neurodevelopmental disorder that exhibits a common set of behavioral and cognitive impairments. Although the etiology of ASD remains unclear, mitochondrial dysfunction has recently emerged as a possible causative factor underlying ASD. The ketogenic diet (KD) is a high-fat, low-carbohydrate diet that augments mitochondrial function, and has been shown to reduce autistic behaviors in both humans and in rodent models of ASD. The aim of the current study was to examine mitochondrial bioenergetics in the BTBR mouse model of ASD and to determine whether the KD improves mitochondrial function. We also investigated changes in mitochondrial morphology, which can directly influence mitochondrial function. We found that BTBR mice had altered mitochondrial function and exhibited smaller more fragmented mitochondria compared to C57BL/6J controls, and that supplementation with the KD improved both mitochondrial function and morphology. We also identified activating phosphorylation of two fission proteins, pDRP1S616 and pMFFS146, in BTBR mice, consistent with the increased mitochondrial fragmentation that we observed. Intriguingly, we found that the KD decreased pDRP1S616 levels in BTBR mice, likely contributing to the restoration of mitochondrial morphology. Overall, these data suggest that impaired mitochondrial bioenergetics and mitochondrial fragmentation may contribute to the etiology of ASD and that these alterations can be reversed with KD treatment.
Keywords: BTBR mouse; autism spectrum disorder; fission; fusion; ketogenic diet; mitochondria; mitochondrial dynamics; mitochondrial function.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
References
-
- Zablotsky B., Black L.I., Maenner M.J., Schieve L.A., Danielson M.L., Bitsko R.H., Blumberg S.J., Kogan M.D., Boyle C.A. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics. 2019;144:e20190811. doi: 10.1542/peds.2019-0811. - DOI - PMC - PubMed

