Gel Formulation of Nabumetone and a Newly Synthesized Analog: Microemulsion as a Photoprotective Topical Delivery System
- PMID: 32380748
- PMCID: PMC7284650
- DOI: 10.3390/pharmaceutics12050423
Gel Formulation of Nabumetone and a Newly Synthesized Analog: Microemulsion as a Photoprotective Topical Delivery System
Abstract
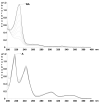
Photostability studies were performed on topical formulations containing the anti-inflammatory drug Nabumetone and an analog newly synthesized in order to achieve better photostability and pharmacokinetic profile. Stability tests, according to the International Conference on Harmonization rules, were applied on ethanol solutions and topical gel formulations of both compounds. The photodegradation profiles were monitored by Multivariate curve resolution applied to the UV spectral data. The inclusion of the compounds in microemulsion was investigated to improve light stability and, at the same time, to ensure a sustained release system for skin delivery. All the formulations in solution, gel, microemulsion, and microemulsion-in-gel were exposed to a forced irradiation of 350 W/m2, corresponding to a 21 kJ/m2 min, for up to 300 min. Photostability increased significantly for both drugs in the liquid microemulsion and microemulsion-in-gel, compared to the ethanol solution and plain gel, reaching a residual drug of 97% and 98% for Nabumetone and analog in microemulsion-in-gel, respectively. Permeation experiments on the microemulsion-in-gel showed a better performance of the analog formulated at 0.2%, compared to the same formulation of Nabumetone at 0.7%. These results highlight the potential of the designed matrices as delayed drug delivery systems along with the use of lower drug doses leading to reduced side effects.
Keywords: anti-inflammatory drugs; microemulsion-in-gel; multivariate curve resolution.; nabumetone; photostability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nobilis M., Mikusek J., Szotakova B., Jirasko R., Holcapek M., Chamseddin C., Jira T., Kucera R., Kunes J., Pour M. Analytical power of LLE-HPLC-PDA-MS/MS in drug metabolism studies: Identification of new nabumetone metabolites. J. Pharmaceut. Biomed. 2013;80:164–172. doi: 10.1016/j.jpba.2013.03.006. - DOI - PubMed
-
- Kawisha S.M., Ahmeda S., Gull A., Aslam M., Pandit J., Aqil M., Sultana Y. Development of nabumetone loaded lipid nano-scaffold for the effective oral delivery; optimization, characterization, drug release and pharmacodynamic study. J. Mol. Liq. 2017;231:514–522. doi: 10.1016/j.molliq.2017.01.107. - DOI
-
- Valero M. Photodegradation of Nabumetone in n-butanol solutions. J. Photoch. Photobio. A. 2004;163:159–164. doi: 10.1016/j.jphotochem.2003.11.005. - DOI
-
- Valero M., Costa Brito S. Photodegradation of Nabumetone in aqueous solutions. J. Photoch. Photobio. A. 2003;157:93–101. doi: 10.1016/S1010-6030(03)00013-3. - DOI
LinkOut - more resources
Full Text Sources

