Induction of Antibacterial Metabolites by Co-Cultivation of Two Red-Sea-Sponge-Associated Actinomycetes Micromonospora sp. UR56 and Actinokinespora sp. EG49
- PMID: 32380771
- PMCID: PMC7281614
- DOI: 10.3390/md18050243
Induction of Antibacterial Metabolites by Co-Cultivation of Two Red-Sea-Sponge-Associated Actinomycetes Micromonospora sp. UR56 and Actinokinespora sp. EG49
Abstract
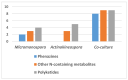
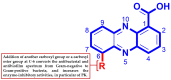
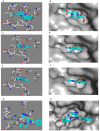
Liquid chromatography coupled with high resolution mass spectrometry (LC-HRESMS)-assisted metabolomic profiling of two sponge-associated actinomycetes, Micromonospora sp. UR56 and Actinokineospora sp. EG49, revealed that the co-culture of these two actinomycetes induced the accumulation of metabolites that were not traced in their axenic cultures. Dereplication suggested that phenazine-derived compounds were the main induced metabolites. Hence, following large-scale co-fermentation, the major induced metabolites were isolated and structurally characterized as the already known dimethyl phenazine-1,6-dicarboxylate (1), phenazine-1,6-dicarboxylic acid mono methyl ester (phencomycin; 2), phenazine-1-carboxylic acid (tubermycin; 3), N-(2-hydroxyphenyl)-acetamide (9), and p-anisamide (10). Subsequently, the antibacterial, antibiofilm, and cytotoxic properties of these metabolites (1-3, 9, and 10) were determined in vitro. All the tested compounds except 9 showed high to moderate antibacterial and antibiofilm activities, whereas their cytotoxic effects were modest. Testing against Staphylococcus DNA gyrase-B and pyruvate kinase as possible molecular targets together with binding mode studies showed that compounds 1-3 could exert their bacterial inhibitory activities through the inhibition of both enzymes. Moreover, their structural differences, particularly the substitution at C-1 and C-6, played a crucial role in the determination of their inhibitory spectra and potency. In conclusion, the present study highlighted that microbial co-cultivation is an efficient tool for the discovery of new antimicrobial candidates and indicated phenazines as potential lead compounds for further development as antibiotic scaffold.
Keywords: DNA gyrase; antibacterial; antibiofilm; co-cultivation; phenazine; pyruvate kinase; sponge-associated actinomycetes.
Conflict of interest statement
The authors declare there is no conflict of interest.
Figures



References
-
- Engelhardt K., Degnes K.F., Kemmler M., Bredholt H., Fjærvik E., Klinkenberg G., Sletta H., Ellingsen T.E., Zotchev S.B. Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl. Environ. Microbiol. 2010;76:4969–4976. doi: 10.1128/AEM.00741-10. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

