Self-analysis of repeat proteins reveals evolutionarily conserved patterns
- PMID: 32381046
- PMCID: PMC7204011
- DOI: 10.1186/s12859-020-3493-y
Self-analysis of repeat proteins reveals evolutionarily conserved patterns
Abstract
Background: Protein repeats can confound sequence analyses because the repetitiveness of their amino acid sequences lead to difficulties in identifying whether similar repeats are due to convergent or divergent evolution. We noted that the patterns derived from traditional "dot plot" protein sequence self-similarity analysis tended to be conserved in sets of related repeat proteins and this conservation could be quantitated using a Jaccard metric.
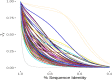
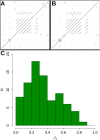
Results: Comparison of these dot plots obviated the issues due to sequence similarity for analysis of repeat proteins. A high Jaccard similarity score was suggestive of a conserved relationship between closely related repeat proteins. The dot plot patterns decayed quickly in the absence of selective pressure with an expected loss of 50% of Jaccard similarity due to a loss of 8.2% sequence identity. To perform method testing, we assembled a standard set of 79 repeat proteins representing all the subgroups in RepeatsDB. Comparison of known repeat and non-repeat proteins from the PDB suggested that the information content in dot plots could be used to identify repeat proteins from pure sequence with no requirement for structural information. Analysis of the UniRef90 database suggested that 16.9% of all known proteins could be classified as repeat proteins. These 13.3 million putative repeat protein chains were clustered and a significant amount (82.9%) of clusters containing between 5 and 200 members were of a single functional type.
Conclusions: Dot plot analysis of repeat proteins attempts to obviate issues that arise due to the sequence degeneracy of repeat proteins. These results show that this kind of analysis can efficiently be applied to analyze repeat proteins on a large scale.
Keywords: Protein evolution; Protein repeat; Repeat identification; Structural bioinformatics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Kidera A, Konishi Y, Ooi T, Scheraga HA. Relation between sequence similarity and structural similarity in proteins - role of important properties of amino-acids. J Protein Chem. 1985;4(5):265–297.
-
- Krissinel E. On the relationship between sequence and structure similarities in proteomics. Bioinformatics. 2007;23(6):717–723. - PubMed
-
- Uversky VN. Intrinsically disordered proteins and their “mysterious” (meta)physics. Front Phys-Lausanne. 2019;7:10.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

