Amorphous SiO2 nanoparticles promote cardiac dysfunction via the opening of the mitochondrial permeability transition pore in rat heart and human cardiomyocytes
- PMID: 32381100
- PMCID: PMC7206702
- DOI: 10.1186/s12989-020-00346-2
Amorphous SiO2 nanoparticles promote cardiac dysfunction via the opening of the mitochondrial permeability transition pore in rat heart and human cardiomyocytes
Abstract
Background: Silica nanoparticles (nanoSiO2) are promising systems that can deliver biologically active compounds to tissues such as the heart in a controllable manner. However, cardiac toxicity induced by nanoSiO2 has been recently related to abnormal calcium handling and energetic failure in cardiomyocytes. Moreover, the precise mechanisms underlying this energetic debacle remain unclear. In order to elucidate these mechanisms, this article explores the ex vivo heart function and mitochondria after exposure to nanoSiO2.
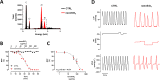
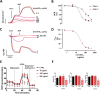
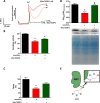
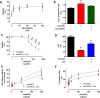
Results: The cumulative administration of nanoSiO2 reduced the mechanical performance index of the rat heart with a half-maximal inhibitory concentration (IC50) of 93 μg/mL, affecting the relaxation rate. In isolated mitochondria nanoSiO2 was found to be internalized, inhibiting oxidative phosphorylation and significantly reducing the mitochondrial membrane potential (ΔΨm). The mitochondrial permeability transition pore (mPTP) was also induced with an increasing dose of nanoSiO2 and partially recovered with, a potent blocker of the mPTP, Cyclosporine A (CsA). The activity of aconitase and thiol oxidation, in the adenine nucleotide translocase, were found to be reduced due to nanoSiO2 exposure, suggesting that nanoSiO2 induces the mPTP via thiol modification and ROS generation. In cardiac cells exposed to nanoSiO2, enhanced viability and reduction of H2O2 were observed after application of a specific mitochondrial antioxidant, MitoTEMPO. Concomitantly, CsA treatment in adult rat cardiac cells reduced the nanoSiO2-triggered cell death and recovered ATP production (from 32.4 to 65.4%). Additionally, we performed evaluation of the mitochondrial effect of nanoSiO2 in human cardiomyocytes. We observed a 40% inhibition of maximal oxygen consumption rate in mitochondria at 500 μg/mL. Under this condition we identified a remarkable diminution in the spare respiratory capacity. This data indicates that a reduction in the amount of extra ATP that can be produced by mitochondria during a sudden increase in energy demand. In human cardiomyocytes, increased LDH release and necrosis were found at increased doses of nanoSiO2, reaching 85 and 48%, respectively. Such deleterious effects were partially prevented by the application of CsA. Therefore, exposure to nanoSiO2 affects cardiac function via mitochondrial dysfunction through the opening of the mPTP.
Conclusion: The aforementioned effects can be partially avoided reducing ROS or retarding the opening of the mPTP. These novel strategies which resulted in cardioprotection could be considered as potential therapies to decrease the side effects of nanoSiO2 exposure.
Keywords: Calcium overload; Cardiotoxicity; Heart; Mitochondria; Oxidative stress; Permeability transition; Silica nanoparticles.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Silica nanoparticles induce cardiotoxicity interfering with energetic status and Ca2+ handling in adult rat cardiomyocytes.Am J Physiol Heart Circ Physiol. 2017 Apr 1;312(4):H645-H661. doi: 10.1152/ajpheart.00564.2016. Epub 2017 Jan 27. Am J Physiol Heart Circ Physiol. 2017. PMID: 28130337 Free PMC article.
-
Antineoplastic copper coordinated complexes (Casiopeinas) uncouple oxidative phosphorylation and induce mitochondrial permeability transition in cardiac mitochondria and cardiomyocytes.J Bioenerg Biomembr. 2016 Feb;48(1):43-54. doi: 10.1007/s10863-015-9640-x. Epub 2016 Jan 7. J Bioenerg Biomembr. 2016. PMID: 26739598
-
Interplay between Ca2+ cycling and mitochondrial permeability transition pores promotes reperfusion-induced injury of cardiac myocytes.J Cell Mol Med. 2011 Nov;15(11):2478-85. doi: 10.1111/j.1582-4934.2010.01249.x. J Cell Mol Med. 2011. PMID: 21199327 Free PMC article.
-
Development or disease: duality of the mitochondrial permeability transition pore.Dev Biol. 2017 Jun 1;426(1):1-7. doi: 10.1016/j.ydbio.2017.04.018. Epub 2017 Apr 28. Dev Biol. 2017. PMID: 28457864 Review.
-
Recent progress in the use of mitochondrial membrane permeability transition pore in mitochondrial dysfunction-related disease therapies.Mol Cell Biochem. 2021 Jan;476(1):493-506. doi: 10.1007/s11010-020-03926-0. Epub 2020 Sep 30. Mol Cell Biochem. 2021. PMID: 33000352 Review.
Cited by
-
Unexpected Inhibitory Role of Silica Nanoparticles on Lung Cancer Development by Promoting M1 Polarization of Macrophages.Int J Nanomedicine. 2024 Nov 1;19:11087-11104. doi: 10.2147/IJN.S472796. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39502640 Free PMC article.
-
Understanding the Mechanism of Cardiotoxicity Induced by Nanomaterials: A Comprehensive Review.Small Sci. 2025 Feb 20;5(5):2400498. doi: 10.1002/smsc.202400498. eCollection 2025 May. Small Sci. 2025. PMID: 40395348 Free PMC article. Review.
-
Cardiomyocyte intercellular signalling increases oxidative stress and reprograms the global- and phospho-proteome of cardiac fibroblasts.J Extracell Biol. 2023 Nov 30;2(12):e125. doi: 10.1002/jex2.125. eCollection 2023 Dec. J Extracell Biol. 2023. PMID: 38938901 Free PMC article.
-
Micro- and Nanosized Substances Cause Different Autophagy-Related Responses.Int J Mol Sci. 2021 Apr 30;22(9):4787. doi: 10.3390/ijms22094787. Int J Mol Sci. 2021. PMID: 33946416 Free PMC article. Review.
-
Mitochondrial Dysfunction in Cardiovascular Diseases: Potential Targets for Treatment.Front Cell Dev Biol. 2022 May 13;10:841523. doi: 10.3389/fcell.2022.841523. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646910 Free PMC article. Review.
References
-
- AB, S. T . TAR Study Investigating Performance and Safety of the Medical Device SiPore15™ [Clinical trial]. Reviewed online from U. S. National Library of Medicine Web page. 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

