Dextran Sulfate Protects Pancreatic β-Cells, Reduces Autoimmunity, and Ameliorates Type 1 Diabetes
- PMID: 32381645
- PMCID: PMC7372066
- DOI: 10.2337/db19-0725
Dextran Sulfate Protects Pancreatic β-Cells, Reduces Autoimmunity, and Ameliorates Type 1 Diabetes
Abstract
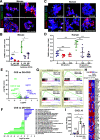
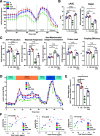
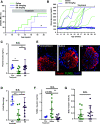
A failure in self-tolerance leads to autoimmune destruction of pancreatic β-cells and type 1 diabetes (T1D). Low-molecular-weight dextran sulfate (DS) is a sulfated semisynthetic polysaccharide with demonstrated cytoprotective and immunomodulatory properties in vitro. However, whether DS can protect pancreatic β-cells, reduce autoimmunity, and ameliorate T1D is unknown. In this study, we report that DS, but not dextran, protects human β-cells against cytokine-mediated cytotoxicity in vitro. DS also protects mitochondrial function and glucose-stimulated insulin secretion and reduces chemokine expression in human islets in a proinflammatory environment. Interestingly, daily treatment with DS significantly reduces diabetes incidence in prediabetic NOD mice and, most importantly, reverses diabetes in early-onset diabetic NOD mice. DS decreases β-cell death, enhances islet heparan sulfate (HS)/HS proteoglycan expression, and preserves β-cell mass and plasma insulin in these mice. DS administration also increases the expression of the inhibitory costimulatory molecule programmed death-1 (PD-1) in T cells, reduces interferon-γ+CD4+ and CD8+ T cells, and enhances the number of FoxP3+ cells. Collectively, these studies demonstrate that the action of one single molecule, DS, on β-cell protection, extracellular matrix preservation, and immunomodulation can reverse diabetes in NOD mice, highlighting its therapeutic potential for the treatment of T1D.
© 2020 by the American Diabetes Association.
Figures



Similar articles
-
Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy.Transplantation. 2009 Jan 27;87(2):198-206. doi: 10.1097/TP.0b013e3181933261. Transplantation. 2009. PMID: 19155973
-
Self-Transducible Bimodal PDX1-FOXP3 Protein Lifts Insulin Secretion and Curbs Autoimmunity, Boosting Tregs in Type 1 Diabetic Mice.Mol Ther. 2018 Jan 3;26(1):184-198. doi: 10.1016/j.ymthe.2017.08.014. Epub 2017 Sep 7. Mol Ther. 2018. PMID: 28988715 Free PMC article.
-
Th1-Like ICOS+ Foxp3+ Treg Cells Preferentially Express CXCR3 and Home to β-Islets during Pre-Diabetes in BDC2.5 NOD Mice.PLoS One. 2015 May 6;10(5):e0126311. doi: 10.1371/journal.pone.0126311. eCollection 2015. PLoS One. 2015. PMID: 25946021 Free PMC article.
-
Chemokines as Drivers of the Autoimmune Destruction in Type 1 Diabetes: Opportunity for Therapeutic Intervention in Consideration of an Optimal Treatment Schedule.Front Endocrinol (Lausanne). 2020 Oct 19;11:591083. doi: 10.3389/fendo.2020.591083. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33193102 Free PMC article. Review.
-
The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis.Ann N Y Acad Sci. 2013 Apr;1281(1):16-35. doi: 10.1111/j.1749-6632.2012.06826.x. Epub 2013 Jan 16. Ann N Y Acad Sci. 2013. PMID: 23323860 Free PMC article. Review.
Cited by
-
Mechanisms and therapeutic strategies of immune checkpoint molecules and regulators in type 1 diabetes.Front Endocrinol (Lausanne). 2023 Jan 10;13:1090842. doi: 10.3389/fendo.2022.1090842. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36704045 Free PMC article. Review.
-
Engineering microparticles based on solidified stem cell secretome with an augmented pro-angiogenic factor portfolio for therapeutic angiogenesis.Bioact Mater. 2022 Apr 2;17:526-541. doi: 10.1016/j.bioactmat.2022.03.015. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35846945 Free PMC article.
-
From Pancreatic β-Cell Gene Networks to Novel Therapies for Type 1 Diabetes.Diabetes. 2021 Sep;70(9):1915-1925. doi: 10.2337/dbi20-0046. Epub 2021 Aug 20. Diabetes. 2021. PMID: 34417266 Free PMC article. Review.
-
Plasma protein biomarkers predict the development of persistent autoantibodies and type 1 diabetes 6 months prior to the onset of autoimmunity.Cell Rep Med. 2023 Jul 18;4(7):101093. doi: 10.1016/j.xcrm.2023.101093. Epub 2023 Jun 29. Cell Rep Med. 2023. PMID: 37390828 Free PMC article.
-
Harmine and exendin-4 combination therapy safely expands human β cell mass in vivo in a mouse xenograft system.Sci Transl Med. 2024 Jul 10;16(755):eadg3456. doi: 10.1126/scitranslmed.adg3456. Epub 2024 Jul 10. Sci Transl Med. 2024. PMID: 38985854 Free PMC article.
References
-
- Coppieters KT, von Herrath MG, Homann D. Autoimmunity and autoimmune diseases. In Fundamental Immunology. 7th ed. Paul W, Ed. Philadelphia, Lippincott Williams & Wilkins, 2013
-
- Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol 2005;87:123–162 - PubMed
-
- Roep BO, Tree TIM. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol 2014;10:229–242 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

