Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice
- PMID: 32381719
- PMCID: PMC7304446
- DOI: 10.1126/science.aaz8899
Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice
Abstract
Loss-of-function mutations in the copper (Cu) transporter ATP7A cause Menkes disease. Menkes is an infantile, fatal, hereditary copper-deficiency disorder that is characterized by progressive neurological injury culminating in death, typically by 3 years of age. Severe copper deficiency leads to multiple pathologies, including impaired energy generation caused by cytochrome c oxidase dysfunction in the mitochondria. Here we report that the small molecule elesclomol escorted copper to the mitochondria and increased cytochrome c oxidase levels in the brain. Through this mechanism, elesclomol prevented detrimental neurodegenerative changes and improved the survival of the mottled-brindled mouse-a murine model of severe Menkes disease. Thus, elesclomol holds promise for the treatment of Menkes and associated disorders of hereditary copper deficiency.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

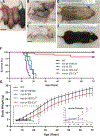


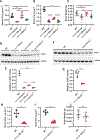
Comment in
-
Sending copper where it is needed most.Science. 2020 May 8;368(6491):584-585. doi: 10.1126/science.abb6662. Science. 2020. PMID: 32381707 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

