ZHX2 drives cell growth and migration via activating MEK/ERK signal and induces Sunitinib resistance by regulating the autophagy in clear cell Renal Cell Carcinoma
- PMID: 32382017
- PMCID: PMC7206010
- DOI: 10.1038/s41419-020-2541-x
ZHX2 drives cell growth and migration via activating MEK/ERK signal and induces Sunitinib resistance by regulating the autophagy in clear cell Renal Cell Carcinoma
Erratum in
-
Correction: ZHX2 drives cell growth and migration via activating MEK/ERK signal and induces Sunitinib resistance by regulating the autophagy in clear cell Renal Cell Carcinoma.Cell Death Dis. 2021 Apr 14;12(4):399. doi: 10.1038/s41419-021-03678-9. Cell Death Dis. 2021. PMID: 33854032 Free PMC article. No abstract available.
Abstract
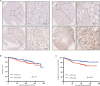
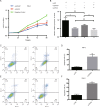
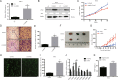
Zinc fingers and homeoboxes 2 (ZHX2) was found as a novel VHL substrate target, and acted as an oncogenic driver in ccRCC. However, the detailed mechanism of ZHX2 in ccRCC development remains elusive, and no research has focused on studying ZHX2 in drug resistance yet. A tissue microarray with 358 ccRCC samples was used to determine the expression of ZHX2 in ccRCC patients. VHL-deficient cell line 786-O and VHL-normal cell line CAKI-1 was used for lineage reprogramming by transfecting with lentivirus. The in vitro and in vivo experiments were performed with these new cell lines to determine the mechanism of ZHX2 in ccRCC development and drug resistance. Immunohistochemistry analysis showed that ZHX2 was not highly expressed in ccRCC tumor tissues, only 33.2% (119/358) patients have high ZHX2 expression. However, high ZHX2 was significantly associated with advanced Fuhrman grade (p = 0.004), and proved to be an independent prognosis factor for progression-free survival (p = 0.0003), while there is no significant correlation with overall survival. We further discovered that ZHX2 overexpression could increase VEGF secretion and transcriptional activate the MEK/ERK1/2 and promote its downstream targets. We also found ZHX2 overexpression induce Sunitinib resistance though activating autophagy and the combination treatment of Sunitinib and Chloroquine could significantly rescue the phenomenon. In summary, these results indicate that ZHX2 drivers cell growth, migration though increase VEGF expression, and transcriptional activate MEK/ERK1/2 signaling pathway, and could induce Sunitinib resistance by regulating self-protective autophagy, these may provide new insight in advanced ccRCC treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

