Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells
- PMID: 32382022
- PMCID: PMC7206079
- DOI: 10.1038/s41419-020-2558-1
Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells
Erratum in
-
Correction to: Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells.Cell Death Dis. 2024 Aug 20;15(8):601. doi: 10.1038/s41419-024-06953-7. Cell Death Dis. 2024. PMID: 39164250 Free PMC article. No abstract available.
Abstract
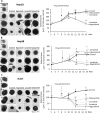
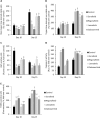
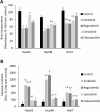
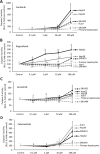
Sorafenib and Regorafenib are the recommended first- and second-line therapies in patients with advanced hepatocellular carcinoma (HCC). Lenvatinib and Cabozantinib have shown non-inferior antitumoral activities compared with the corresponding recommended therapies. The clinical trials have established recommended doses for each treatment that lead different blood concentrations in patients for Sorafenib (10 µM), Regorafenib (1 µM), Lenvatinib (0.1 µM), and Cabozantinib (1 µM). However, very low response rates are observed in patients attributed to intrinsic resistances or upregulation of survival signaling. The aim of the study was the comparative dose-response analysis of the drugs (0-100 µM) in well-differentiated (HepG2, Hep3B, and Huh7), moderately (SNU423), and poorly (SNU449) differentiated liver cancer cells in 2D/3D cultures. Cells harbors wild-type p53 (HepG2), non-sense p53 mutation (Hep3B), inframe p53 gene deletion (SNU423), and p53 point mutation (Huh7 and SNU449). The administration of regular used in vitro dose (10 µM) in 3D and 2D cultures, as well as the dose-response analysis in 2D cultures showed Sorafenib and Regorafenib were increasingly effective in reducing cell proliferation, and inducing apoptosis in well-differentiated and expressing wild-type p53 in HCC cells. Lenvatinib and Cabozantinib were particularly effective in moderately to poorly differentiated cells with mutated or lacking p53 that have lower basal oxygen consumption rate (OCR), ATP, and maximal respiration capacity than observed in differentiated HCC cells. Sorafenib and Regorafenib downregulated, and Lenvatinib and Cabozantinib upregulated epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor receptor (c-Met) in HepG2 cells. Conclusions: Sorafenib and Regorafenib were especially active in well-differentiated cells, with wild-type p53 and increased mitochondrial respiration. By contrast, Lenvatinib and Cabozantinib appeared more effective in moderately to poorly differentiated cells with mutated p53 and low mitochondrial respiration. The development of strategies that allow us to deliver increased doses in tumors might potentially enhance the effectiveness of the treatments.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Different Mechanisms of Action of Regorafenib and Lenvatinib on Toll-Like Receptor-Signaling Pathways in Human Hepatoma Cell Lines.Int J Mol Sci. 2020 May 9;21(9):3349. doi: 10.3390/ijms21093349. Int J Mol Sci. 2020. PMID: 32397371 Free PMC article.
-
Cabozantinib inhibits the growth of lenvatinib-resistant hepatoma cells restoring FTCD expression.Biochem Pharmacol. 2024 Aug;226:116321. doi: 10.1016/j.bcp.2024.116321. Epub 2024 May 28. Biochem Pharmacol. 2024. PMID: 38815631
-
Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma.Adv Ther. 2020 Jun;37(6):2678-2695. doi: 10.1007/s12325-020-01378-y. Epub 2020 May 18. Adv Ther. 2020. PMID: 32424805 Free PMC article.
-
Molecular therapies for HCC: Looking outside the box.J Hepatol. 2020 Feb;72(2):342-352. doi: 10.1016/j.jhep.2019.09.010. J Hepatol. 2020. PMID: 31954496 Review.
-
Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma.World J Gastroenterol. 2019 Aug 7;25(29):3870-3896. doi: 10.3748/wjg.v25.i29.3870. World J Gastroenterol. 2019. PMID: 31413525 Free PMC article. Review.
Cited by
-
Genetic Heterogeneity, Therapeutic Hurdle Confronting Sorafenib and Immune Checkpoint Inhibitors in Hepatocellular Carcinoma.Cancers (Basel). 2021 Aug 27;13(17):4343. doi: 10.3390/cancers13174343. Cancers (Basel). 2021. PMID: 34503153 Free PMC article. Review.
-
Modelling hepatocellular carcinoma microenvironment phenotype to evaluate drug efficacy.Sci Rep. 2025 Jan 7;15(1):1179. doi: 10.1038/s41598-024-84304-4. Sci Rep. 2025. PMID: 39774014 Free PMC article.
-
Enhancement of apoptosis in HCT116 and HepG2 cells by Coix lacryma-jobi var. lacryma-jobi seed extract in combination with sorafenib.Chin Herb Med. 2025 Feb 21;17(2):322-339. doi: 10.1016/j.chmed.2025.02.005. eCollection 2025 Apr. Chin Herb Med. 2025. PMID: 40256710 Free PMC article.
-
Cellular Adaptation Takes Advantage of Atavistic Regression Programs during Carcinogenesis.Cancers (Basel). 2023 Aug 3;15(15):3942. doi: 10.3390/cancers15153942. Cancers (Basel). 2023. PMID: 37568758 Free PMC article. Review.
-
Three-Dimensional Hepatocyte Spheroids: Model for Assessing Chemotherapy in Hepatocellular Carcinoma.Biomedicines. 2024 May 28;12(6):1200. doi: 10.3390/biomedicines12061200. Biomedicines. 2024. PMID: 38927406 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

