SHMT inhibition is effective and synergizes with methotrexate in T-cell acute lymphoblastic leukemia
- PMID: 32382081
- PMCID: PMC7647950
- DOI: 10.1038/s41375-020-0845-6
SHMT inhibition is effective and synergizes with methotrexate in T-cell acute lymphoblastic leukemia
Abstract
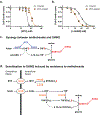
Folate metabolism enables cell growth by providing one-carbon (1C) units for nucleotide biosynthesis. The 1C units are carried by tetrahydrofolate, whose production by the enzyme dihydrofolate reductase is targeted by the important anticancer drug methotrexate. 1C units come largely from serine catabolism by the enzyme serine hydroxymethyltransferase (SHMT), whose mitochondrial isoform is strongly upregulated in cancer. Here we report the SHMT inhibitor SHIN2 and demonstrate its in vivo target engagement with 13C-serine tracing. As methotrexate is standard treatment for T-cell acute lymphoblastic leukemia (T-ALL), we explored the utility of SHIN2 in this disease. SHIN2 increases survival in NOTCH1-driven mouse primary T-ALL in vivo. Low dose methotrexate sensitizes Molt4 human T-ALL cells to SHIN2, and cells rendered methotrexate resistant in vitro show enhanced sensitivity to SHIN2. Finally, SHIN2 and methotrexate synergize in mouse primary T-ALL and in a human patient-derived xenograft in vivo, increasing survival. Thus, SHMT inhibition offers a complementary strategy in the treatment of T-ALL.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R00 CA197869/CA/NCI NIH HHS/United States
- 1DP1DK113643/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30CA072720/Rutgers Cancer Institute of New Jersey (Cancer Institute of New Jersey)
- R01CA163591/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RSG-19-161-01-TBE/American Cancer Society (American Cancer Society, Inc.)
- R00CA215307/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 CA236936/CA/NCI NIH HHS/United States
- DP1 DK113643/DK/NIDDK NIH HHS/United States
- P30 CA072720/CA/NCI NIH HHS/United States
- R01CA236936/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R00 CA215307/CA/NCI NIH HHS/United States
- R01 CA163591/CA/NCI NIH HHS/United States
- R00CA197869/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Research Materials

