High-risk additional chromosomal abnormalities at low blast counts herald death by CML
- PMID: 32382082
- PMCID: PMC7387244
- DOI: 10.1038/s41375-020-0826-9
High-risk additional chromosomal abnormalities at low blast counts herald death by CML
Erratum in
-
Correction: High-risk additional chromosomal abnormalities at low blast counts herald death by CML.Leukemia. 2020 Oct;34(10):2823. doi: 10.1038/s41375-020-01039-7. Leukemia. 2020. PMID: 32913312 Free PMC article.
Abstract
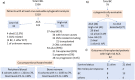
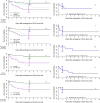
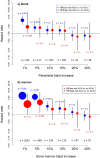
Blast crisis is one of the remaining challenges in chronic myeloid leukemia (CML). Whether additional chromosomal abnormalities (ACAs) enable an earlier recognition of imminent blastic proliferation and a timelier change of treatment is unknown. One thousand five hundred and ten imatinib-treated patients with Philadelphia-chromosome-positive (Ph+) CML randomized in CML-study IV were analyzed for ACA/Ph+ and blast increase. By impact on survival, ACAs were grouped into high risk (+8, +Ph, i(17q), +17, +19, +21, 3q26.2, 11q23, -7/7q abnormalities; complex) and low risk (all other). The presence of high- and low-risk ACAs was linked to six cohorts with different blast levels (1%, 5%, 10%, 15%, 20%, and 30%) in a Cox model. One hundred and twenty-three patients displayed ACA/Ph+ (8.1%), 91 were high risk. At low blast levels (1-15%), high-risk ACA showed an increased hazard to die compared to no ACA (ratios: 3.65 in blood; 6.12 in marrow) in contrast to low-risk ACA. No effect was observed at blast levels of 20-30%. Sixty-three patients with high-risk ACA (69%) died (n = 37) or were alive after progression or progression-related transplantation (n = 26). High-risk ACA at low blast counts identify end-phase CML earlier than current diagnostic systems. Mortality was lower with earlier treatment. Cytogenetic monitoring is indicated when signs of progression surface or response to therapy is unsatisfactory.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Anastasi J, Feng J, Le Beau MM, Larson RA, Rowley JD, Vardiman JW. The relationship between secondary chromosomal abnormalities and blast transformation in chronic myelogenous leukemia. Leukemia. 1995;9:628–33. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

