Hydrocortisone and bronchopulmonary dysplasia: variables associated with response in premature infants
- PMID: 32382114
- PMCID: PMC7222054
- DOI: 10.1038/s41372-020-0680-7
Hydrocortisone and bronchopulmonary dysplasia: variables associated with response in premature infants
Abstract
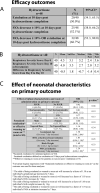
Objective: The primary objective was to evaluate hydrocortisone's efficacy for decreasing respiratory support in premature infants with developing bronchopulmonary dysplasia (BPD). Secondary objectives included assessment of the impact of intrauterine growth restriction (IUGR), maternal history of chorioamnionitis, side effects and route of administration associated with hydrocortisone's efficacy. Dexamethasone as second-line treatment to decrease respiratory support was reviewed.
Methods: Retrospective chart review of preterm infants requiring respiratory support receiving hydrocortisone.
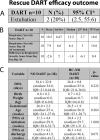
Results: A total of 48 patients were included. Successful extubation was achieved in 50% of intubated patients after hydrocortisone treatment with no major complications. In our small study, history of maternal chorioamnionitis, IUGR or route of administration did not affect the response. Rescue dexamethasone after hydrocortisone therapy was ineffective in the ten patients who failed extubation following hydrocortisone.
Conclusion: Hydrocortisone is effective in decreasing respiratory support in patients with developing BPD without major complications. Randomized studies are warranted to confirm our findings.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics. 1990;86:728–36. - PubMed
-
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

