Predictive value of tumor genetic alteration profiling for chemotherapy and EGFR-TKI treatment in advanced NSCLC
- PMID: 32382334
- PMCID: PMC7202306
- DOI: 10.3892/ol.2020.11502
Predictive value of tumor genetic alteration profiling for chemotherapy and EGFR-TKI treatment in advanced NSCLC
Abstract
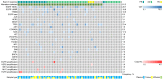
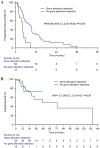
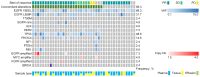
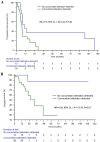
Previous studies have suggested that a variety of tumor driver genetic alterations affected the treatment efficacy of chemotherapy and epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) in advanced non-small cell lung cancer (NSCLC). The present study aimed to investigate the association between the tumor genetic alteration landscape and the treatment outcome of first-line chemotherapy and EGFR-TKIs in advanced NSCLC. A total of 94 patients with advanced NSCLC were recruited. All patients received first-line chemotherapy and/or EGFR-TKIs (either first- or second-generation EGFR-TKI, or third-generation EGFR-TKI) alone or sequentially. Prior to chemotherapy and/or EGFR-TKI treatment, plasma, effusion and/or tumor tissues from the included patients were subjected to next-generation sequencing, targeting 59 genes. The results indicated that the positive genetic alteration status prior to first-line chemotherapy was associated with prolonged progression-free survival (PFS) time compared with the negative status [9.1 vs. 4.0 months; hazard ratio (HR)=6.68; 95% CI, 2.25-19.82; P=0.001). Furthermore, patients with EGFR activating mutation harboring concomitant alterations exhibited a shorter PFS (11.1 vs. 7.4 months; HR=2.14; 95% CI, 1.03-4.44; P=0.04) and overall survival (OS) time [not reached (NR) vs. 32.8 months; HR=4.30; 95% CI, 1.41-13.16; P=0.01] than those without concomitant alterations, with first- and second-generation EGFR-TKI treatment. Similarly, patients with T79M mutation harboring concomitant alterations exhibited a shorter PFS (15.6 vs. 3.6 months; HR=9.48; 95% CI, 2.29-39.28; P=0.002) and OS time (NR vs. 32.8 months; HR=4.85; 95% CI, 1.16-20.29; P=0.03) with osimertinib treatment. Taken together, the results demonstrated that positive genetic alteration status predicted greater efficacy of first-line chemotherapy, while concomitant genetic alterations were associated with poor treatment outcome for first- or second-generation EGFR-TKI and third-generation EGFR-TKI treatment.
Keywords: EGFR-TKI; NGS; NSCLC; chemotherapy; tumor genetic alteration profiling.
Copyright: © Jiang et al.
Figures

Similar articles
-
Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis.JAMA. 2014 Apr 9;311(14):1430-7. doi: 10.1001/jama.2014.3314. JAMA. 2014. PMID: 24715074
-
[The concomitant gene alterations impact the therapeutic efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer patients with epidermal growth factor receptor sensitive mutation].Zhonghua Jie He He Hu Xi Za Zhi. 2018 Oct 12;41(10):778-782. doi: 10.3760/cma.j.issn.1001-0939.2018.10.006. Zhonghua Jie He He Hu Xi Za Zhi. 2018. PMID: 30347549 Chinese.
-
The Efficacy of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer Harboring Wild-type Epidermal Growth Factor Receptor: A Meta-analysis of 25 RCTs.Am J Clin Oncol. 2017 Aug;40(4):362-369. doi: 10.1097/COC.0000000000000179. Am J Clin Oncol. 2017. PMID: 25647830
-
Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival.J Natl Cancer Inst. 2017 Jun 1;109(6). doi: 10.1093/jnci/djw279. J Natl Cancer Inst. 2017. PMID: 28376144 Review.
-
Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC).Lung Cancer. 2019 Nov;137:113-122. doi: 10.1016/j.lungcan.2019.09.017. Epub 2019 Sep 23. Lung Cancer. 2019. PMID: 31568888 Free PMC article. Review.
Cited by
-
Clinical and Molecular Features of Epidermal Growth Factor Receptor (EGFR) Mutation Positive Non-Small-Cell Lung Cancer (NSCLC) Patients Treated with Tyrosine Kinase Inhibitors (TKIs): Predictive and Prognostic Role of Co-Mutations.Cancers (Basel). 2021 May 17;13(10):2425. doi: 10.3390/cancers13102425. Cancers (Basel). 2021. PMID: 34067823 Free PMC article.
References
-
- Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y, Fukuoka M. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm cooperative study in Japan. Ann Oncol. 2007;18:317–323. doi: 10.1093/annonc/mdl377. - DOI - PubMed
-
- Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna A, Fidias P, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. doi: 10.1200/JCO.2003.12.046. - DOI - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-I patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. - DOI - PubMed
-
- Rodrigues-Pereira J, Kim JH, Magallanes M, Lee DH, Wang J, Ganju V, Martinez-Barrera L, Barraclough H, van Kooten M, Orlando M. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:1907–1914. doi: 10.1097/JTO.0b013e318226b5fa. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
