Sleep, circadian rhythms and health
- PMID: 32382406
- PMCID: PMC7202392
- DOI: 10.1098/rsfs.2019.0098
Sleep, circadian rhythms and health
Abstract
At the core of human thought, for the majority of individuals in the developed nations at least, there is the tacit assumption that as a species we are unfettered by the demands imposed by our biology and that we can do what we want, at whatever time we choose, whereas in reality every aspect of our physiology and behaviour is constrained by a 24 h beat arising from deep within our evolution. Our daily circadian rhythms and sleep/wake cycle allow us to function optimally in a dynamic world, adjusting our biology to the demands imposed by the day/night cycle. The themes developed in this review focus upon the growing realization that we ignore the circadian and sleep systems at our peril, and this paper considers the mechanisms that generate and regulate circadian and sleep systems; what happens mechanistically when these systems collapse as a result of societal pressures and disease; how sleep disruption and stress are linked; why sleep disruption and mental illness invariably occur together; and how individuals and employers can attempt to mitigate some of the problems associated with working against our internal temporal biology. While some of the health costs of sleep disruption can be reduced, in the short-term at least, there will always be significant negative consequences associated with shift work and sleep loss. With this in mind, society needs to address this issue and decide when the consequences of sleep disruption are justified in the workplace.
Keywords: CBTi; circadian; health; insomnia; sleep; stress.
© 2020 The Author(s).
Conflict of interest statement
I declare I have no competing interests.
Figures
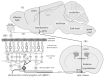


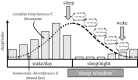




References
-
- Foster RG, Kreitzman L. 2004. Rhythms of life: the biological clocks that control the daily lives of every living thing, xii, 276 pp London, UK: Profile Books.
-
- Lockley SW, Foster RG. 2012. Sleep: a very short introduction, xvi, 146 pp Oxford, NY: Oxford University Press.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
