Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis
- PMID: 32382566
- PMCID: PMC7180397
- DOI: 10.1155/2020/7091718
Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis
Abstract
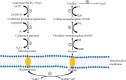
Pyrimidine nucleoside uridine plays a critical role in maintaining cellular function and energy metabolism. In addition to its role in nucleoside synthesis, uridine and its derivatives contribute to reduction of cytotoxicity and suppression of drug-induced hepatic steatosis. Uridine is mostly present in blood and cerebrospinal fluid, where it contributes to the maintenance of basic cellular functions affected by UPase enzyme activity, feeding habits, and ATP depletion. Uridine metabolism depends on three stages: de novo synthesis, salvage synthesis pathway and catabolism, and homeostasis, which is tightly relating to glucose homeostasis and lipid and amino acid metabolism. This review is devoted to uridine metabolism and its role in glucose, lipid, and amino acid homeostasis.
Copyright © 2020 Yumei Zhang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

