Magnetic Nanoparticles as MRI Contrast Agents
- PMID: 32382832
- PMCID: PMC8203530
- DOI: 10.1007/s41061-020-00302-w
Magnetic Nanoparticles as MRI Contrast Agents
Erratum in
-
Correction to: Magnetic Nanoparticles as MRI Contrast Agents.Top Curr Chem (Cham). 2021 Jun 14;379(4):30. doi: 10.1007/s41061-021-00340-y. Top Curr Chem (Cham). 2021. PMID: 34128112 Free PMC article. No abstract available.
Abstract
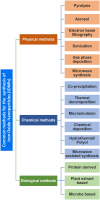
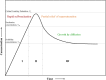
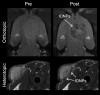
Iron oxide nanoparticles (IONPs) have emerged as a promising alternative to conventional contrast agents (CAs) for magnetic resonance imaging (MRI). They have been extensively investigated as CAs due to their high biocompatibility and excellent magnetic properties. Furthermore, the ease of functionalization of their surfaces with different types of ligands (antibodies, peptides, sugars, etc.) opens up the possibility of carrying out molecular MRI. Thus, IONPs functionalized with epithelial growth factor receptor antibodies, short peptides, like RGD, or aptamers, among others, have been proposed for the diagnosis of various types of cancer, including breast, stomach, colon, kidney, liver or brain cancer. In addition to cancer diagnosis, different types of IONPs have been developed for other applications, such as the detection of brain inflammation or the early diagnosis of thrombosis. This review addresses key aspects in the development of IONPs for MRI applications, namely, synthesis of the inorganic core, functionalization processes to make IONPs biocompatible and also to target them to specific tissues or cells, and finally in vivo studies in animal models, with special emphasis on tumor models.
Keywords: Cancer; Diagnosis; Iron oxide nanoparticles; Magnetic nanoparticles; Magnetic resonance imaging.
Figures



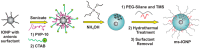
Similar articles
-
Biodistribution and Tumors MRI Contrast Enhancement of Magnetic Nanocubes, Nanoclusters, and Nanorods in Multiple Mice Models.Contrast Media Mol Imaging. 2018 Sep 24;2018:8264208. doi: 10.1155/2018/8264208. eCollection 2018. Contrast Media Mol Imaging. 2018. PMID: 30344459 Free PMC article.
-
New Insight about Biocompatibility and Biodegradability of Iron Oxide Magnetic Nanoparticles: Stereological and In Vivo MRI Monitor.Sci Rep. 2019 May 9;9(1):7173. doi: 10.1038/s41598-019-43650-4. Sci Rep. 2019. PMID: 31073222 Free PMC article.
-
Biocompatible Peptide-Coated Ultrasmall Superparamagnetic Iron Oxide Nanoparticles for In Vivo Contrast-Enhanced Magnetic Resonance Imaging.ACS Nano. 2018 Jul 24;12(7):6480-6491. doi: 10.1021/acsnano.7b07572. Epub 2018 Jul 11. ACS Nano. 2018. PMID: 29979569
-
Iron oxide nanoparticles - In vivo/in vitro biomedical applications and in silico studies.Adv Colloid Interface Sci. 2017 Nov;249:192-212. doi: 10.1016/j.cis.2017.05.003. Epub 2017 May 3. Adv Colloid Interface Sci. 2017. PMID: 28499604 Review.
-
Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI.Chem Soc Rev. 2015 Oct 7;44(19):6733-48. doi: 10.1039/c5cs00331h. Chem Soc Rev. 2015. PMID: 26169237 Review.
Cited by
-
Nanomaterials: leading immunogenic cell death-based cancer therapies.Front Immunol. 2024 Aug 9;15:1447817. doi: 10.3389/fimmu.2024.1447817. eCollection 2024. Front Immunol. 2024. PMID: 39185425 Free PMC article. Review.
-
In situ embedding dual-Fe nanoparticles in synchronously generated carbon for the synergistic integration of magnetic resonance imaging and drug delivery.Nanoscale Adv. 2020 Sep 26;2(11):5296-5304. doi: 10.1039/d0na00714e. eCollection 2020 Nov 11. Nanoscale Adv. 2020. PMID: 36132027 Free PMC article.
-
Iron-Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy.Pharmaceutics. 2022 Mar 14;14(3):636. doi: 10.3390/pharmaceutics14030636. Pharmaceutics. 2022. PMID: 35336012 Free PMC article.
-
Magnetite Nanoparticles: Synthesis and Applications in Optics and Nanophotonics.Materials (Basel). 2022 Apr 1;15(7):2601. doi: 10.3390/ma15072601. Materials (Basel). 2022. PMID: 35407934 Free PMC article. Review.
-
Nanoparticle-Based Techniques for Bladder Cancer Imaging: A Review.Int J Mol Sci. 2023 Feb 14;24(4):3812. doi: 10.3390/ijms24043812. Int J Mol Sci. 2023. PMID: 36835222 Free PMC article. Review.
References
-
- García-Martín ML, López-Larrubia P. Preclinical MRI: methods and protocols, vol 1718. Methods in molecular biology. New York: Springer; 2018.
-
- Leong HS, Butler KS, Brinker CJ, Azzawi M, Conlan S, Dufés C, Owen A, Rannard S, Scott C, Chen C, Dobrovolskaia MA, Kozlov SV, Prina-Mello A, Schmid R, Wick P, Caputo F, Boisseau P, Crist RM, McNeil SE, Fadeel B, Tran L, Hansen SF, Hartmann NB, Clausen LPW, Skjolding LM, Baun A, Ågerstrand M, Gu Z, Lamprou DA, Hoskins C, Huang L, Song W, Cao H, Liu X, Jandt KD, Jiang W, Kim BYS, Wheeler KE, Chetwynd AJ, Lynch I, Moghimi SM, Nel A, Xia T, Weiss PS, Sarmento B, das Neves J, Santos HA, Santos L, Mitragotri S, Little S, Peer D, Amiji MM, Alonso MJ, Petri-Fink A, Balog S, Lee A, Drasler B, Rothen-Rutishauser B, Wilhelm S, Acar H, Harrison RG, Mao C, Mukherjee P, Ramesh R, McNally LR, Busatto S, Wolfram J, Bergese P, Ferrari M, Fang RH, Zhang L, Zheng J, Peng C, Du B, Yu M, Charron DM, Zheng G, Pastore C. On the issue of transparency and reproducibility in nanomedicine. Nat Nanotechnol. 2019;14(7):629–635. doi: 10.1038/s41565-019-0496-9. - DOI - PMC - PubMed
-
- Zhi D, Yang T, Yang J, Fu S, Zhang S (2020) Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater 102:13–34. 10.1016/j.actbio.2019.11.027 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
