Distribution patterns of tau pathology in progressive supranuclear palsy
- PMID: 32383020
- PMCID: PMC7360645
- DOI: 10.1007/s00401-020-02158-2
Distribution patterns of tau pathology in progressive supranuclear palsy
Abstract
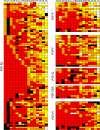
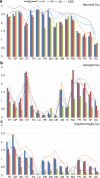
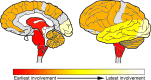
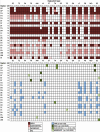
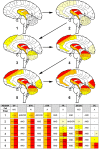
Progressive supranuclear palsy (PSP) is a 4R-tauopathy predominated by subcortical pathology in neurons, astrocytes, and oligodendroglia associated with various clinical phenotypes. In the present international study, we addressed the question of whether or not sequential distribution patterns can be recognized for PSP pathology. We evaluated heat maps and distribution patterns of neuronal, astroglial, and oligodendroglial tau pathologies and their combinations in different clinical subtypes of PSP in postmortem brains. We used conditional probability and logistic regression to model the sequential distribution of tau pathologies across different brain regions. Tau pathology uniformly predominates in the neurons of the pallido-nigro-luysian axis in different clinical subtypes. However, clinical subtypes are distinguished not only by total tau load but rather cell-type (neuronal versus glial) specific vulnerability patterns of brain regions suggesting distinct dynamics or circuit-specific segregation of propagation of tau pathologies. For Richardson syndrome (n = 81) we recognize six sequential steps of involvement of brain regions by the combination of cellular tau pathologies. This is translated to six stages for the practical neuropathological diagnosis by the evaluation of the subthalamic nucleus, globus pallidus, striatum, cerebellum with dentate nucleus, and frontal and occipital cortices. This system can be applied to further clinical subtypes by emphasizing whether they show caudal (cerebellum/dentate nucleus) or rostral (cortical) predominant, or both types of pattern. Defining cell-specific stages of tau pathology helps to identify preclinical or early-stage cases for the better understanding of early pathogenic events, has implications for understanding the clinical subtype-specific dynamics of disease-propagation, and informs tau-neuroimaging on distribution patterns.
Keywords: Coiled body; Neurofibrillary tangle; Progressive supranuclear palsy; Propagation; Richardson syndrome; Sequential involvement; Stage; Tau; Tauopathy; Tufted astrocyte.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

