Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples
- PMID: 32383254
- PMCID: PMC7267461
- DOI: 10.1002/jmv.25986
Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples
Abstract
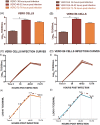
The micro-neutralization assay is a fundamental test in virology, immunology, vaccine assessment, and epidemiology studies. Since the SARS-CoV-2 outbreak at the end of December 2019 in China, it has become extremely important to have well-established and validated diagnostic and serological assays for this new emerging virus. Here, we present a micro-neutralization assay with the use of SARS-CoV-2 wild type virus with two different methods of read-out. We evaluated the performance of this assay using human serum samples taken from an Italian seroepidemiological study being performed at the University of Siena, along with the human monoclonal antibody CR3022 and some iper-immune animal serum samples against Influenza and Adenovirus strains. The same panel of human samples have been previously tested in enzyme-linked immunosorbent assay (ELISA) as a pre-screening. Positive, borderline, and negative ELISA samples were evaluated in neutralization assay using two different methods of read-out: subjective (by means of an inverted optical microscope) and objective (by means of a spectrophotometer). Our findings suggest that at least 50% of positive ELISA samples are positive in neutralization as well, and that method is able to quantify different antibody concentrations in a specific manner. Taken together, our results confirm that the colorimetric cytopathic effect-based microneutralization assay could be used as a valid clinical test method for epidemiological and vaccine studies.
Keywords: SARS coronavirus; epidemiology; humoral immunity; neutralization; pandemic.
© 2020 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures


References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

