Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline
- PMID: 32383274
- PMCID: PMC7891343
- DOI: 10.1111/jcpe.13290
Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline
Erratum in
-
Corrigendum.J Clin Periodontol. 2021 Jan;48(1):163. doi: 10.1111/jcpe.13403. J Clin Periodontol. 2021. PMID: 33370480 Free PMC article. No abstract available.
Abstract
Background: The recently introduced 2017 World Workshop on the classification of periodontitis, incorporating stages and grades of disease, aims to link disease classification with approaches to prevention and treatment, as it describes not only disease severity and extent but also the degree of complexity and an individual's risk. There is, therefore, a need for evidence-based clinical guidelines providing recommendations to treat periodontitis.
Aim: The objective of the current project was to develop a S3 Level Clinical Practice Guideline (CPG) for the treatment of Stage I-III periodontitis.
Material and methods: This S3 CPG was developed under the auspices of the European Federation of Periodontology (EFP), following the methodological guidance of the Association of Scientific Medical Societies in Germany and the Grading of Recommendations Assessment, Development and Evaluation (GRADE). The rigorous and transparent process included synthesis of relevant research in 15 specifically commissioned systematic reviews, evaluation of the quality and strength of evidence, the formulation of specific recommendations and consensus, on those recommendations, by leading experts and a broad base of stakeholders.
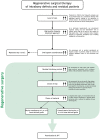
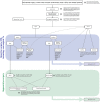
Results: The S3 CPG approaches the treatment of periodontitis (stages I, II and III) using a pre-established stepwise approach to therapy that, depending on the disease stage, should be incremental, each including different interventions. Consensus was achieved on recommendations covering different interventions, aimed at (a) behavioural changes, supragingival biofilm, gingival inflammation and risk factor control; (b) supra- and sub-gingival instrumentation, with and without adjunctive therapies; (c) different types of periodontal surgical interventions; and (d) the necessary supportive periodontal care to extend benefits over time.
Conclusion: This S3 guideline informs clinical practice, health systems, policymakers and, indirectly, the public on the available and most effective modalities to treat periodontitis and to maintain a healthy dentition for a lifetime, according to the available evidence at the time of publication.
Keywords: clinical guideline; grade; health policy; oral health; periodontal therapy; periodontitis; stage.
© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Workshop participants filed detailed disclosure of potential conflict of interest relevant to the workshop topics, and these are kept on file. Declared potential dual commitments included having received research funding, consultant fees and speaker fee from the industries with economic interests in the interventions for prevention and therapy of Periodontitis. Those affected with potential conflict of interest abstained from vote in the specific recommendations following the required processes for S3 level clinical practice guideline. Individual potential conflict of interest forms were completed by all participants and are available on file at the European Federation of Periodontology and extracted in the Supporting Information, available online (Final Guideline‐Supporting Information_Potential conflict of interests). In addition, potential conflict of interest information of the chairs of the workshop is listed here.
Figures
Comment in
-
EVIDENCE-BASED CLINICAL PRACTICE GUIDELINE FOR TREATMENT OF STAGE IIII PERIODONTITIS.J Evid Based Dent Pract. 2021 Dec;21(4):101638. doi: 10.1016/j.jebdp.2021.101638. Epub 2021 Aug 30. J Evid Based Dent Pract. 2021. PMID: 34922721
References
-
- Abouassi, T. , Woelber, J. , Holst, K. , Stampf, S. , Doerfer, C. , Hellwig, E. , & Ratka‐Krüger, P. (2014). Clinical efficacy and patients' acceptance of a rubber interdental bristle. A randomized controlled trial. Clinical Oral Investigations, 18(7), 1873–1880. 10.1007/s00784-013-1164-3 - DOI - PubMed
-
- Araujo, A. A. , Pereira, A. , Medeiros, C. , Brito, G. A. C. , Leitao, R. F. C. , Araujo, L. S. , … Araujo Junior, R. F. (2017). Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS ONE, 12(8), e0183506 10.1371/journal.pone.0183506 - DOI - PMC - PubMed
-
- Badran, Z. , Kraehenmann, M. A. , Guicheux, J. , & Soueidan, A. (2009). Bisphosphonates in periodontal treatment: A review. Oral Health and Preventive Dentistry, 7(1), 3–12. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical