A biallelic pathogenic variant in the OGDH gene results in a neurological disorder with features of a mitochondrial disease
- PMID: 32383294
- PMCID: PMC7647956
- DOI: 10.1002/jimd.12248
A biallelic pathogenic variant in the OGDH gene results in a neurological disorder with features of a mitochondrial disease
Abstract
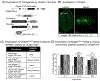
2-Oxoglutarate dehydrogenase (OGDH) is a rate-limiting enzyme in the mitochondrial TCA cycle, encoded by the OGDH gene. α-Ketoglutarate dehydrogenase (OGDH) deficiency was previously reported in association with developmental delay, hypotonia, and movement disorders and metabolic decompensation, with no genetic data provided. Using whole exome sequencing, we identified two individuals carrying a homozygous missense variant c.959A>G (p.N320S) in the OGDH gene. These individuals presented with global developmental delay, elevated lactate, ataxia and seizure. Fibroblast analysis and modeling of the mutation in Drosophila were used to evaluate pathogenicity of the variant. Skin fibroblasts from subject # 2 showed a decrease in both OGDH protein and enzyme activity. Transfection of human OGDH cDNA in HEK293 cells carrying p.N320S also produced significantly lower protein levels compared to those with wild-type cDNA. Loss of Drosophila Ogdh (dOgdh) caused early developmental lethality, rescued by expressing wild-type dOgdh (dOgdhWT ) or human OGDH (OGDHWT ) cDNA. In contrast, expression to the mutant OGDH (OGDHN320S ) or dOgdh carrying homologous mutations to human OGDH p.N320S variant (dOgdhN324S ) failed to rescue lethality of dOgdh null mutants. Knockdown of dOgdh in the nervous system resulted in locomotion defects which were rescued by dOgdhWT expression but not by dOgdhN324S expression. Collectively, the results indicate that c.959A>G variant in OGDH leads to an amino acid change (p.N320S) causing a severe loss of OGDH protein function. Our study establishes in the first time a genetic link between an OGDH gene mutation and OGDH deficiency.
Keywords: OGDH; TCA cycle; alpha-ketoglutarate dehydrogenase deficiency; genetic disease.
© 2020 SSIEM.
Conflict of interest statement
CONFLICT OF INTEREST
Zheng Yie Yap, Klaudia Strucinska, Satoshi Matsuzaki, Sukyeong Lee, Yue Si, Kenneth Humphries, Mark A. Tarnopolsky, and Wan Hee Yoon declare that they have no conflicts of interest in the publication of this article. Yue Si is an employee of GeneDx, Inc.
Figures



References
-
- Metodiev MD, Gerber S, Hubert L, et al. Mutations in the tricarboxylic acid cycle enzyme, aconitase 2, cause either isolated or syndromic optic neuropathy with encephalopathy and cerebellar atrophy. J Med Genet. 2014;51:834–838. - PubMed
-
- Ostergaard E, Hansen FJ, Sorensen N, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. 2007;130:853–861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

